Metabotropic Glutamate Receptor 7: From Synaptic Function to Therapeutic Implications
- PMID: 27306064
- PMCID: PMC4983754
- DOI: 10.2174/1570159x13666150716165323
Metabotropic Glutamate Receptor 7: From Synaptic Function to Therapeutic Implications
Abstract
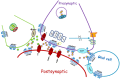
Metabotropic glutamate receptor 7 (mGluR7) is localized presynaptically at the active zone of neurotransmitter release. Unlike mGluR4 and mGluR8, which share mGluR7's presynaptic location, mGluR7 shows low affinity for glutamate and is activated only by high glutamate concentrations. Its wide distribution in the central nervous system (CNS) and evolutionary conservation across species suggest that mGluR7 plays a primary role in controlling excitatory synapse function. High mGluR7 expression has been observed in several brain regions that are critical for CNS functioning and are involved in neurological and psychiatric disorder development. Until the recent discovery of selective ligands for mGluR7, techniques to elucidate its role in neural function were limited to the use of knockout mice and gene silencing. Studies using these two techniques have revealed that mGluR7 modulates emotionality, stress and fear responses. N,N`-dibenzhydrylethane-1,2-diamine dihydrochloride (AMN082) was reported as the first selective mGluR7 allosteric agonist. Pharmacological effects of AMN082 have not completely confirmed the mGluR7-knockout mouse phenotype; this has been attributed to rapid receptor internalization after drug treatment and to the drug's apparent lack of in vivo selectivity. Therefore, the more recently developed mGluR7 negative allosteric modulators (NAMs) are crucial for understanding mGluR7 function and for exploiting its potential as a target for therapeutic interventions. This review presents the main findings regarding mGluR7's effect on modulation of synaptic function and its role in normal CNS function and in models of neurologic and psychiatric disorders.
Conflict of interest statement
All the authors have read and approved the paper and have not any financial or other relationships that might lead to a conflict of interest.
Figures
References
-
- Conn P.J., Pin J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. [http://dx.doi.org/10.1146/annurev.pharmtox.37.1.205]. [PMID: 9131252]. - PubMed
-
- Swanson C.J., Bures M., Johnson M.P., Linden A.M., Monn J.A., Schoepp D.D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005;4(2):131–144. [http://dx.doi.org/10.1038/nrd1630]. [PMID: 15665858]. - PubMed
-
- Ferraguti F., Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326(2):483–504. [http://dx.doi.org/10.1007/ s00441-006-0266-5]. [PMID: 16847639]. - PubMed
-
- Ritter S.L., Hall R.A. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 2009;10(12):819–830. [http://dx.doi.org/10.1038/nrm2803]. [PMID: 19935667]. - PMC - PubMed
-
- Shigemoto R., Kulik A., Roberts J.D., Ohishi H., Nusser Z., Kaneko T., Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381(6582):523–525. [http://dx.doi.org/10.1038/ 381523a0]. [PMID: 8632825]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
