Ziprasidone augmentation for anxious depression
- PMID: 27306192
- PMCID: PMC5039050
- DOI: 10.1097/YIC.0000000000000133
Ziprasidone augmentation for anxious depression
Abstract
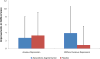
Previously, we found an anxiolytic effect of ziprasidone augmentation to escitalopram (compared with placebo augmentation) in patients with depression in an 8-week, randomized, double-blind, parallel-group, placebo-controlled trial. Here, we carried out a post-hoc analysis, comparing changes in the Hamilton Depression and Anxiety Rating Scales between patients with anxious depression versus nonanxious depression, using a moderator analysis. Hamilton Depression Rating Scales total change scores from baseline and endpoint were not significantly different (interaction term P=0.91) in patients with anxious depression on ziprasidone augmentation (n=19; -9.1±4.9) or placebo (n=19; -6.1±8.9) versus patients without anxious depression on ziprasidone (n=52; -5.5±6.7) or placebo (n=49; -2.3±4.5). There was a trend toward statistical significance (interaction term P=0.1) in favor of patients without anxious depression for a difference in Hamilton Anxiety Rating Scale total change scores from baseline to endpoint [patients with anxious depression on ziprasidone augmentation (n=19; -2.7±5.3) or placebo (n=19; -3.3±5.8) versus patients without anxious depression on ziprasidone (n=51; -3.9±6.6) or placebo (n=44; -0.9±4.7)]. Ziprasidone augmentation was equally efficacious in treating depression in patients with versus without anxious depression. However, the observed anxiolytic effect for patients with higher anxiety was not clinically significant.
Trial registration: ClinicalTrials.gov NCT00633399.
Conflict of interest statement
Conflicts and Disclosures: George I. Papakostas: Consultant: Abbott Laboratories, AstraZeneca PLC, Avanir Pharmaceuticals, Brainsway Ltd, Bristol-Myers Squibb Company, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., GlaxoSmithKline, Evotec AG, H. Lundbeck A/S, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, Novartis Pharma AG, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Laboratories, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., and Wyeth, Inc.; Grant/Research Support: AstraZeneca PLC, Bristol-Myers Squibb Company, Forest Pharmaceuticals, the National Institute of Mental Health, PAMLAB LLC, Pfizer Inc., Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sunovion Pharmaceuticals, and Theracos, Inc.; Honoraria (for consulting or educational activities): Abbott Laboratories, Astra Zeneca PLC, Avanir Pharmaceuticals, Bristol-Myers Squibb Company, Brainsway Ltd, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Evotec AG, GlaxoSmithKline, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, H. Lundbeck A/S, Novartis Pharma AG, Otsuka Pharmaceuticals, Pamlab LLC, Pfizer, Pierre Fabre Laboratories, Ridge Diagnostics, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., Titan Pharmaceuticals, and Wyeth Inc.; Speaker or Advisory Boards: BristolMyersSquibb Co and Pfizer, Inc. (Previous). This study was supported by the National Institute for Mental Health (NIMH R01MH081235), Pfizer Inc. (providing free blinded ziprasidone/placebo pills), and Forest Laboratories, Inc. (providing free escitalopram). Richard C. Shelton: Consultant: Cerecor, Inc., Clintara, LLC, Janssen Pharmaceutica, MSI Methylation Sciences, Inc., Naurex, Inc., Nestle’ Health-Pamlab, Inc., Pfizer, Inc., Ridge Diagnostics. Grant/research support: Alkermes, Inc., Assurex Health, Avanir Pharmaceuticals, Forest Pharmaceuticals (Allergan), Genomind, Janssen Pharmaceutica, Johnson & Johnson, Naurex Inc., Novartis Inc., Otsuka Pharmaceuticals, Nestle’ Health-Pamlab, Inc., Takeda Pharmaceuticals. Dawn F. Ionescu: Received research funding from a Young Investigator Award through the Brain and Behavior Research Foundation, KL2/CMeRIT Award from the Harvard Catalyst, K23 award from the National Institute for Mental Health (1K23MH107776-01), and from the Executive Committee on Research at Massachusetts General Hospital.
Figures

References
-
- BANDELOW B, BAUER M, VIETA E, EL-KHALILI N, GUSTAFSSON U, EARLEY WR, ERIKSSON H. Extended release quetiapine fumarate as adjunct to antidepressant therapy in patients with major depressive disorder: pooled analyses of data in patients with anxious depression versus low levels of anxiety at baseline. World J Biol Psychiatry. 2014;15:155–66. - PubMed
-
- CHAN HN, RUSH AJ, NIERENBERG AA, TRIVEDI M, WISNIEWSKI SR, BALASUBRAMANI GK, FRIEDMAN ES, GAYNES BN, DAVIS L, MORRIS D, FAVA M. Correlates and outcomes of depressed out-patients with greater and fewer anxious symptoms: a CO-MED report. Int J Neuropsychopharmacol. 2012;15:1387–99. - PubMed
-
- CLEARY P, GUY W. Factor analysis of Hamilton depression scale. Drugs Exp Clin Res. 1977:115–120.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

