Hypothalamic CRH neurons orchestrate complex behaviours after stress
- PMID: 27306314
- PMCID: PMC4912635
- DOI: 10.1038/ncomms11937
Hypothalamic CRH neurons orchestrate complex behaviours after stress
Abstract
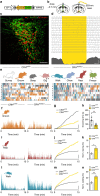
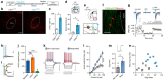
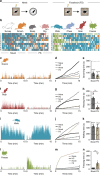
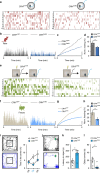
All organisms possess innate behavioural and physiological programmes that ensure survival. In order to have maximum adaptive benefit, these programmes must be sufficiently flexible to account for changes in the environment. Here we show that hypothalamic CRH neurons orchestrate an environmentally flexible repertoire of behaviours that emerge after acute stress in mice. Optical silencing of CRH neurons disrupts the organization of individual behaviours after acute stress. These behavioural patterns shift according to the environment after stress, but this environmental sensitivity is blunted by activation of PVN CRH neurons. These findings provide evidence that PVN CRH cells are part of a previously unexplored circuit that matches precise behavioural patterns to environmental context following stress. Overactivity in this network in the absence of stress may contribute to environmental ambivalence, resulting in context-inappropriate behavioural strategies.
Figures



References
-
- Cannon W. B. Bodily Changes in Pain, Hunger, Fear, and Rage Appleton (1915).
-
- Selye H. A syndrome produced by diverse nocuous agents. Nature 138, 32 (1936). - PubMed
-
- Blanchard D. C., Blanchard R. J. & Griebel G. Defensive responses to predator threat in the rat and mouse. Curr. Protoc. Neurosci Chapter 8, Unit 8.19 (2005). - PubMed
-
- Blanchard D. C., Griebel G., Pobbe R. & Blanchard R. J. Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev. 35, 991–998 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

