EXPRESS: Methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: Effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn
- PMID: 27306413
- PMCID: PMC4956006
- DOI: 10.1177/1744806916657089
EXPRESS: Methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: Effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn
Abstract
Background: Vincristine, a widely used chemotherapeutic agent, often induces painful peripheral neuropathy and there are currently no effective drugs to prevent or treat this side effect. Previous studies have shown that methylcobalamin has potential analgesic effect in diabetic and chronic compression of dorsal root ganglion model; however, whether methylcobalamin has effect on vincristine-induced painful peripheral neuropathy is still unknown.
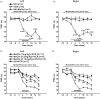
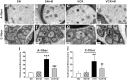
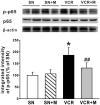
Results: We found that vincristine-induced mechanical allodynia and thermal hyperalgesia, accompanied by a significant loss of intraepidermal nerve fibers in the plantar hind paw skin and an increase in the incidence of atypical mitochondria in the sciatic nerve. Moreover, in the spinal dorsal horn, the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the protein expression of p-p65 as well as tumor necrosis factor a was increased, whereas the protein expression of IL-10 was decreased following vincristine treatment. Furthermore, intraperitoneal injection of methylcobalamin could dose dependently attenuate vincristine-induced mechanical allodynia and thermal hyperalgesia, which was associated with intraepidermal nerve fibers rescue, and atypical mitochondria prevalence decrease in the sciatic nerve. Moreover, methylcobalamin inhibited the activation of NADPH oxidase and the downstream NF-kB pathway. Production of tumor necrosis factor a was also decreased and production of IL-10 was increased in the spinal dorsal horn following methylcobalamin treatment. Intrathecal injection of Phorbol-12-Myristate-13-Acetate, a NADPH oxidase activator, could completely block the analgesic effect of methylcobalamin.
Conclusions: Methylcobalamin attenuated vincrinstine-induced neuropathic pain, which was accompanied by inhibition of intraepidermal nerve fibers loss and mitochondria impairment. Inhibiting the activation of NADPH oxidase and the downstream NF-kB pathway, resulting in the rebalancing of proinflammatory and anti-inflammatory cytokines in the spinal dorsal horn might also be involved. These findings might provide potential target for preventing vincristine-induced neuropathic pain.
Figures




References
-
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol 2002; 249: 9–17. - PubMed
-
- Sisignano M, Baron R, Scholich K, et al. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol 2014; 10: 694–707. - PubMed
-
- Wolf S, Barton D, Kottschade L, et al. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 2008; 44: 1507–1515. - PubMed
-
- Pachman DR, Watson JC, Loprinzi CL. Therapeutic strategies for cancer treatment related peripheral neuropathies. Curr Treat Options Oncol 2014; 15: 567–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

