Identification of structurally closely related monosaccharide and disaccharide isomers by PMP labeling in conjunction with IM-MS/MS
- PMID: 27306514
- PMCID: PMC4910106
- DOI: 10.1038/srep28079
Identification of structurally closely related monosaccharide and disaccharide isomers by PMP labeling in conjunction with IM-MS/MS
Abstract
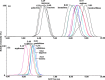
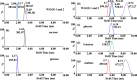
It remains particularly difficult for gaining unambiguous information on anomer, linkage, and position isomers of oligosaccharides using conventional mass spectrometry (MS) methods. In our laboratory, an ion mobility (IM) shift strategy was employed to improve confidence in the identification of structurally closely related disaccharide and monosaccharide isomers using IMMS. Higher separation between structural isomers was achieved using 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatization in comparison with phenylhydrazine (PHN) derivatization. Furthermore, the combination of pre-IM fragmentation of PMP derivatives provided sufficient resolution to separate the isomers not resolved in the IMMS. To chart the structural variation observed in IMMS, the collision cross sections (CCSs) for the corresponding ions were measured. We analyzed nine disaccharide and three monosaccharide isomers that differ in composition, linkages, or configuration. Our data show that coexisting carbohydrate isomers can be identified by the PMP labeling technique in conjunction with ion-mobility separation and tandem mass spectrometry. The practical application of this rapid and effective method that requires only small amounts of sample is demonstrated by the successful analysis of water-soluble ginseng extract. This demonstrated the potential of this method to measure a variety of heterogeneous sample mixtures, which may have an important impact on the field of glycomics.
Figures




References
-
- Zhang X. L. Roles of glycans and glycopeptides in immune system and immune-related diseases. Curr. Med. Chem. 13, 1141–1147 (2006). - PubMed
-
- Rudd P. M., Elliott T., Cresswell P., Wilson I. A. & Dwek R. A. Glycosylation and the immune system. Science 291, 2370−2376 (2001). - PubMed
-
- Pabst M. & Altmann F. Glycan analysis by modern instrumental methods. Proteomics 11, 631−643 (2011). - PubMed
-
- Fang T. T. & Bendiak B. The stereochemical dependence of unimolecular dissociation of monosaccharide-glycolaldehyde anions in the gas phase: a basis for assignment of the stereochemistry and anomeric configuration of monosaccharides in oligosaccharides by mass spectrometry via a key discriminatory product ion of disaccharide fragmentation, m/z 221. J. Am. Chem. Soc. 129, 9721−9736 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

