IgE-mediated enhancement of CD4(+) T cell responses requires antigen presentation by CD8α(-) conventional dendritic cells
- PMID: 27306570
- PMCID: PMC4910288
- DOI: 10.1038/srep28290
IgE-mediated enhancement of CD4(+) T cell responses requires antigen presentation by CD8α(-) conventional dendritic cells
Abstract
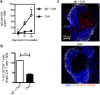
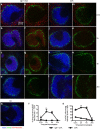
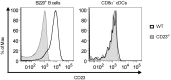
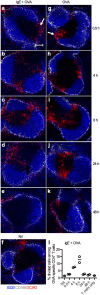
IgE, forming an immune complex with small proteins, can enhance the specific antibody and CD4(+) T cell responses in vivo. The effects require the presence of CD23 (Fcε-receptor II)(+) B cells, which capture IgE-complexed antigens (Ag) in the circulation and transport them to splenic B cell follicles. In addition, also CD11c(+) cells, which do not express CD23, are required for IgE-mediated enhancement of T cell responses. This suggests that some type of dendritic cell obtains IgE-Ag complexes from B cells and presents antigenic peptides to T cells. To elucidate the nature of this dendritic cell, mice were immunized with ovalbumin (OVA)-specific IgE and OVA, and different populations of CD11c(+) cells, obtained from the spleens four hours after immunization, were tested for their ability to present OVA. CD8α(-) conventional dendritic cells (cDCs) were much more efficient in inducing specific CD4(+) T cell proliferation ex vivo than were CD8α(+) cDCs or plasmacytoid dendritic cells. Thus, IgE-Ag complexes administered intravenously are rapidly transported to the spleen by recirculating B cells where they are delivered to CD8α(-) cDCs which induce proliferation of CD4(+) T cells.
Figures

Similar articles
-
Antibody feedback regulation.Immunol Rev. 2024 Nov;328(1):126-142. doi: 10.1111/imr.13377. Epub 2024 Aug 23. Immunol Rev. 2024. PMID: 39180190 Free PMC article. Review.
-
IgE-mediated enhancement of CD4+ T cell responses in mice requires antigen presentation by CD11c+ cells and not by B cells.PLoS One. 2011;6(7):e21760. doi: 10.1371/journal.pone.0021760. Epub 2011 Jul 6. PLoS One. 2011. PMID: 21765910 Free PMC article.
-
A novel B cell-mediated transport of IgE-immune complexes to the follicle of the spleen.J Immunol. 2008 May 15;180(10):6604-10. doi: 10.4049/jimmunol.180.10.6604. J Immunol. 2008. PMID: 18453579
-
Antigen conjugated to anti-CD23 antibodies is rapidly transported to splenic follicles by recirculating B cells.Scand J Immunol. 2015 Jan;81(1):39-45. doi: 10.1111/sji.12248. Scand J Immunol. 2015. PMID: 25359575
-
Role of mouse dendritic cell subsets in priming naive CD4 T cells.Curr Opin Immunol. 2023 Aug;83:102352. doi: 10.1016/j.coi.2023.102352. Epub 2023 Jun 3. Curr Opin Immunol. 2023. PMID: 37276821 Free PMC article. Review.
Cited by
-
On the complexity of IgE: The role of structural flexibility and glycosylation for binding its receptors.Front Allergy. 2023 Mar 28;4:1117611. doi: 10.3389/falgy.2023.1117611. eCollection 2023. Front Allergy. 2023. PMID: 37056355 Free PMC article. Review.
-
IgG3-antigen complexes are deposited on follicular dendritic cells in the presence of C1q and C3.Sci Rep. 2017 Jul 14;7(1):5400. doi: 10.1038/s41598-017-05704-3. Sci Rep. 2017. PMID: 28710441 Free PMC article.
-
Antibody feedback regulation.Immunol Rev. 2024 Nov;328(1):126-142. doi: 10.1111/imr.13377. Epub 2024 Aug 23. Immunol Rev. 2024. PMID: 39180190 Free PMC article. Review.
-
Immunoglobulin E-Dependent Activation of Immune Cells in Rhinovirus-Induced Asthma Exacerbation.Front Allergy. 2022 Feb 22;3:835748. doi: 10.3389/falgy.2022.835748. eCollection 2022. Front Allergy. 2022. PMID: 35386658 Free PMC article.
-
Therapeutic monoclonal antibodies in allergy: Targeting IgE, cytokine, and alarmin pathways.Immunol Rev. 2024 Nov;328(1):387-411. doi: 10.1111/imr.13380. Epub 2024 Aug 19. Immunol Rev. 2024. PMID: 39158477 Free PMC article. Review.
References
-
- Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol. 18, 709–737 (2000). - PubMed
-
- Heyman B., Tianmin L. & Gustavsson S. In vivo enhancement of the specific antibody response via the low-affinity receptor for IgE. Eur. J. Immunol. 23, 1739–1742 (1993). - PubMed
-
- Gustavsson S., Hjulström S., Liu T. & Heyman B. CD23/IgE-mediated regulation of the specific antibody response in vivo. J. Immunol. 152, 4793–4800 (1994). - PubMed
-
- Getahun A., Hjelm F. & Heyman B. IgE enhances antibody and T cell responses in vivo via CD23+ B cells. J. Immunol. 175, 1473–1482 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

