Tau physiology and pathomechanisms in frontotemporal lobar degeneration
- PMID: 27306859
- PMCID: PMC5094566
- DOI: 10.1111/jnc.13600
Tau physiology and pathomechanisms in frontotemporal lobar degeneration
Abstract
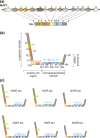
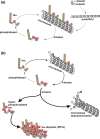
Frontotemporal lobar degeneration (FTLD) has been associated with toxic intracellular aggregates of hyperphosphorylated tau (FTLD-tau). Moreover, genetic studies identified mutations in the MAPT gene encoding tau in familial cases of the disease. In this review, we cover a range of aspects of tau function, both in the healthy and diseased brain, discussing several in vitro and in vivo models. Tau structure and function in the healthy brain is presented, accentuating its distinct compartmentalization in neurons and its role in microtubule stabilization and axonal transport. Furthermore, tau-driven pathology is discussed, introducing current concepts and the underlying experimental evidence. Different aspects of pathological tau phosphorylation, the protein's genomic and domain organization as well as its spreading in disease, together with MAPT-associated mutations and their respective models are presented. Dysfunction related to other post-transcriptional modifications and their effect on normal neuronal functions such as cell cycle, epigenetics and synapse dynamics are also discussed, providing a mechanistic explanation for the observations made in FTLD-tau cases, with the possibility for therapeutic intervention. In this review, we cover aspects of tau function, both in the healthy and diseased brain, referring to different in vitro and in vivo models. In healthy neurons, tau is compartmentalized, with higher concentrations found in the distal part of the axon. Cargo molecules are sensitive to this gradient. A disturbed tau distribution, as found in frontotemporal lobar degeneration (FTLD-tau), has severe consequences for cellular physiology: tau accumulates in the neuronal soma and dendrites, leading among others to microtubule depolymerization and impaired axonal transport. Tau forms insoluble aggregates that sequester additional molecules stalling cellular physiology. Neuronal communication is gradually lost as toxic tau accumulates in dendritic spines with subsequent degeneration of synapses and synaptic loss. Thus, by providing a mechanistic explanation for the observations made in FTLD-tau cases, arises a possibility for therapeutic interventions. This article is part of the Frontotemporal Dementia special issue.
Keywords: microtubule; phosphorylation; post-translational; spreading; synapse; transgenic.
© 2016 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures

References
-
- Alonso A. C., Grundke‐Iqbal I. and Iqbal K. (1996) Alzheimer's disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 2, 783–787. - PubMed
-
- Alzheimer A. (1907) Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z Psychiat. 64, 146–148.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

