Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events
- PMID: 27306911
- PMCID: PMC4943391
- DOI: 10.1634/theoncologist.2015-0509
Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events
Abstract
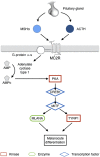
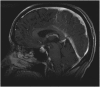
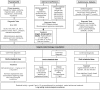
: In recent years, immune checkpoint inhibitors have emerged as effective therapies for advanced neoplasias. As new checkpoint target blockers become available and additional tumor locations tested, their use is expected to increase within a short time. Immune-related adverse events (irAEs) affecting the endocrine system are among the most frequent and complex toxicities. Some may be life-threatening if not recognized; hence, appropriate guidance for oncologists is needed. Despite their high incidence, endocrine irAEs have not been fully described for all immunotherapy agents available. This article is a narrative review of endocrinopathies associated with cytotoxic T lymphocyte-associated antigen-4, blockade of programmed death receptor 1 and its ligand inhibitors, and their combination. Thyroid dysfunction is the most frequent irAE reported, and hypophysitis is characteristic of ipilimumab. Incidence, timing patterns, and clinical presentation are discussed, and practical recommendations for clinical management are suggested. Heterogeneous terminology and lack of appropriate resolution criteria in clinical trials make adequate evaluation of endocrine AEs difficult. It is necessary to standardize definitions to contrast incidences and characterize toxicity patterns. To provide optimal care, a multidisciplinary team that includes endocrinology specialists is recommended.
Implications for practice: Immune checkpoint inhibitors are already part of oncologists' therapeutic arsenal as effective therapies for otherwise untreatable neoplasias, such as metastatic melanoma or lung cancer. Their use is expected to increase exponentially in the near future as additional agents become available and their approval is extended to different tumor types. Adverse events affecting the endocrine system are among the most frequent and complex toxicities oncologists may face, and some may be life-threatening if not recognized. This study reviews endocrinopathies associated to immune checkpoint inhibitors available to date. Incidence, timing patterns, and clinical presentation are discussed, and practical recommendations for management are proposed.
Keywords: Autoimmune hypophysitis; Cytotoxic T-lymphocyte antigen 4; Monoclonal antibodies; Programmed cell death 1 receptor; Thyroiditis.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
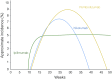
- Pennock GK, Waterfield W, Wolchok JD. Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: How different are these from conventional treatment responses? Am J Clin Oncol. 2012;35:606–611. - PubMed
-
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

