Microglial Signaling in Chronic Pain with a Special Focus on Caspase 6, p38 MAP Kinase, and Sex Dependence
- PMID: 27307048
- PMCID: PMC5004238
- DOI: 10.1177/0022034516653604
Microglial Signaling in Chronic Pain with a Special Focus on Caspase 6, p38 MAP Kinase, and Sex Dependence
Abstract
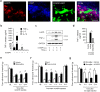
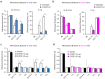
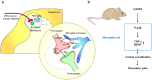
Microglia are the resident immune cells in the spinal cord and brain. Mounting evidence suggests that activation of microglia plays an important role in the pathogenesis of chronic pain, including chronic orofacial pain. In particular, microglia contribute to the transition from acute pain to chronic pain, as inhibition of microglial signaling reduces pathologic pain after inflammation, nerve injury, and cancer but not baseline pain. As compared with inflammation, nerve injury induces much more robust morphologic activation of microglia, termed microgliosis, as shown by increased expression of microglial markers, such as CD11b and IBA1. However, microglial signaling inhibitors effectively reduce inflammatory pain and neuropathic pain, arguing against the importance of morphologic activation of microglia in chronic pain sensitization. Importantly, microglia enhance pain states via secretion of proinflammatory and pronociceptive mediators, such as tumor necrosis factor α, interleukins 1β and 18, and brain-derived growth factor. Mechanistically, these mediators have been shown to enhance excitatory synaptic transmission and suppress inhibitory synaptic transmission in the pain circuits. While early studies suggested a predominant role of microglia in the induction of chronic pain, further studies have supported a role of microglia in the maintenance of chronic pain. Intriguingly, recent studies show male-dominant microglial signaling in some neuropathic pain and inflammatory pain states, although both sexes show identical morphologic activation of microglia after nerve injury. In this critical review, we provide evidence to show that caspase 6-a secreted protease that is expressed in primary afferent axonal terminals surrounding microglia-is a robust activator of microglia and induces profound release of tumor necrosis factor α from microglia via activation of p38 MAP kinase. The authors also show that microglial caspase 6/p38 signaling is male dominant in some inflammatory and neuropathic pain conditions. Finally, the authors discuss the relevance of microglial signaling in chronic trigeminal and orofacial pain.
Keywords: microglia; orofacial pain; sex-related; spinal cord; trigeminal pain; tumor necrosis factor.
© International & American Associations for Dental Research 2016.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures

References
-
- Callahan BL, Gil AS, Levesque A, Mogil JS. 2008. Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J Pain. 9(2):174–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

