The Human Retrosplenial Cortex and Thalamus Code Head Direction in a Global Reference Frame
- PMID: 27307227
- PMCID: PMC5321500
- DOI: 10.1523/JNEUROSCI.1268-15.2016
The Human Retrosplenial Cortex and Thalamus Code Head Direction in a Global Reference Frame
Abstract
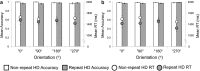
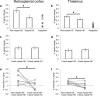
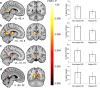
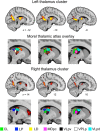
Spatial navigation is a multisensory process involving integration of visual and body-based cues. In rodents, head direction (HD) cells, which are most abundant in the thalamus, integrate these cues to code facing direction. Human fMRI studies examining HD coding in virtual environments (VE) have reported effects in retrosplenial complex and (pre-)subiculum, but not the thalamus. Furthermore, HD coding appeared insensitive to global landmarks. These tasks, however, provided only visual cues for orientation, and attending to global landmarks did not benefit task performance. In the present study, participants explored a VE comprising four separate locales, surrounded by four global landmarks. To provide body-based cues, participants wore a head-mounted display so that physical rotations changed facing direction in the VE. During subsequent MRI scanning, subjects saw stationary views of the environment and judged whether their orientation was the same as in the preceding trial. Parameter estimates extracted from retrosplenial cortex and the thalamus revealed significantly reduced BOLD responses when HD was repeated. Moreover, consistent with rodent findings, the signal did not continue to adapt over repetitions of the same HD. These results were supported by a whole-brain analysis showing additional repetition suppression in the precuneus. Together, our findings suggest that: (1) consistent with the rodent literature, the human thalamus may integrate visual and body-based, orientation cues; (2) global reference frame cues can be used to integrate HD across separate individual locales; and (3) immersive training procedures providing full body-based cues may help to elucidate the neural mechanisms supporting spatial navigation.
Significance statement: In rodents, head direction (HD) cells signal facing direction in the environment via increased firing when the animal assumes a certain orientation. Distinct brain regions, the retrosplenial cortex (RSC) and thalamus, code for visual and vestibular cues of orientation, respectively. Putative HD signals have been observed in human RSC but not the thalamus, potentially because body-based cues were not provided. Here, participants encoded HD in a novel virtual environment while wearing a head-mounted display to provide body-based cues for orientation. In subsequent fMRI scanning, we found evidence of an HD signal in RSC, thalamus, and precuneus. These findings harmonize rodent and human data, and suggest that immersive training procedures provide a viable way to examine the neural basis of navigation.
Keywords: fMRI; head direction; human; navigation; retrosplenial cortex; thalamus.
Copyright © 2016 the authors 0270-6474/16/366371-11$15.00/0.
Figures


Similar articles
-
Environmental Anchoring of Head Direction in a Computational Model of Retrosplenial Cortex.J Neurosci. 2016 Nov 16;36(46):11601-11618. doi: 10.1523/JNEUROSCI.0516-16.2016. J Neurosci. 2016. PMID: 27852770 Free PMC article.
-
A model of head direction and landmark coding in complex environments.PLoS Comput Biol. 2021 Sep 27;17(9):e1009434. doi: 10.1371/journal.pcbi.1009434. eCollection 2021 Sep. PLoS Comput Biol. 2021. PMID: 34570749 Free PMC article.
-
Encoding of 3D head direction information in the human brain.Hippocampus. 2019 Jul;29(7):619-629. doi: 10.1002/hipo.23060. Epub 2018 Dec 18. Hippocampus. 2019. PMID: 30561118 Free PMC article.
-
Thalamocortical processing of the head-direction sense.Prog Neurobiol. 2019 Dec;183:101693. doi: 10.1016/j.pneurobio.2019.101693. Epub 2019 Sep 21. Prog Neurobiol. 2019. PMID: 31550513 Review.
-
The retrosplenial-parietal network and reference frame coordination for spatial navigation.Behav Neurosci. 2018 Oct;132(5):416-429. doi: 10.1037/bne0000260. Epub 2018 Aug 9. Behav Neurosci. 2018. PMID: 30091619 Free PMC article. Review.
Cited by
-
A short review on emotion processing: a lateralized network of neuronal networks.Brain Struct Funct. 2022 Mar;227(2):673-684. doi: 10.1007/s00429-021-02331-7. Epub 2021 Jul 3. Brain Struct Funct. 2022. PMID: 34216271 Free PMC article. Review.
-
Spatial navigation and memory: A review of the similarities and differences relevant to brain models and age.Neuron. 2023 Apr 5;111(7):1037-1049. doi: 10.1016/j.neuron.2023.03.001. Neuron. 2023. PMID: 37023709 Free PMC article. Review.
-
Grid-cell representations in mental simulation.Elife. 2016 Aug 30;5:e17089. doi: 10.7554/eLife.17089. Elife. 2016. PMID: 27572056 Free PMC article.
-
Contextual memory engrams, and the neuromodulatory influence of the locus coeruleus.Front Mol Neurosci. 2024 Feb 5;17:1342622. doi: 10.3389/fnmol.2024.1342622. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38375501 Free PMC article. Review.
-
Improving cognitive mapping by training for people with a poor sense of direction.Cogn Res Princ Implic. 2020 Aug 17;5(1):39. doi: 10.1186/s41235-020-00238-1. Cogn Res Princ Implic. 2020. PMID: 32804308 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
