Strength of ERK1/2 MAPK Activation Determines Its Effect on Myelin and Axonal Integrity in the Adult CNS
- PMID: 27307235
- PMCID: PMC5015783
- DOI: 10.1523/JNEUROSCI.0299-16.2016
Strength of ERK1/2 MAPK Activation Determines Its Effect on Myelin and Axonal Integrity in the Adult CNS
Abstract
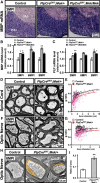
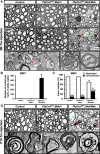
Myelin growth is a tightly regulated process driven by multiple signals. ERK1/2-MAPK signaling is an important regulator of myelin thickness. Because, in demyelinating diseases, the myelin formed during remyelination fails to achieve normal thickness, increasing ERK1/2 activity in oligodendrocytes is of obvious therapeutic potential for promoting efficient remyelination. However, other studies have suggested that increased levels of ERK1/2 activity could, in fact, have detrimental effects on myelinating cells. Because the strength, duration, or timing of ERK1/2 activation may alter the biological outcomes of cellular responses markedly, here, we investigated the effect of modulating ERK1/2 activity in myelinating cells using transgenic mouse lines in which ERK1/2 activation was upregulated conditionally in a graded manner. We found enhanced myelin gene expression and myelin growth in the adult CNS at both moderate and hyperactivated levels of ERK1/2 when upregulation commenced during developmental myelination or was induced later during adulthood in quiescent preexisting oligodendrocytes, after active myelination is largely terminated. However, a late onset of demyelination and axonal degeneration occurred at hyperelevated, but not moderately elevated, levels regardless of the timing of the upregulation. Similarly, myelin and axonal pathology occurred with elevated ERK1/2 activity in Schwann cells. We conclude that a fine tuning of ERK1/2 signaling strength is critically important for normal oligodendrocyte and Schwann cell function and that disturbance of this balance has negative consequences for myelin and axonal integrity in the long term. Therefore, therapeutic modulation of ERK1/2 activity in demyelinating disease or peripheral neuropathies must be approached with caution.
Significance statement: ERK1/2-MAPK activation in oligodendrocytes and Schwann cells is an important signal for promoting myelin growth during developmental myelination. Here, we show that, when ERK1/2 are activated in mature quiescent oligodendrocytes during adulthood, new myelin growth is reinitiated even after active myelination is terminated, which has implications for understanding the mechanism underlying plasticity of myelin in adult life. Paradoxically, simply increasing the "strength" of ERK1/2 activation changed the biological outcome from beneficial to detrimental, adversely affecting myelin and axonal integrity in both the CNS and PNS. Therefore, this study highlights the complexity of ERK1/2-MAPK signaling in the context of oligodendrocyte and Schwann cell function in the adult animal and emphasizes the need to approach potential therapeutic modulation of ERK1/2 activity with caution.
Keywords: Schwann cells; myelin; oligodendrocyte.
Copyright © 2016 the authors 0270-6474/16/366471-17$15.00/0.
Figures








Similar articles
-
ERK1/2 Activation in Preexisting Oligodendrocytes of Adult Mice Drives New Myelin Synthesis and Enhanced CNS Function.J Neurosci. 2016 Aug 31;36(35):9186-200. doi: 10.1523/JNEUROSCI.1444-16.2016. J Neurosci. 2016. PMID: 27581459 Free PMC article.
-
ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination.J Neurosci. 2012 Jun 27;32(26):8855-64. doi: 10.1523/JNEUROSCI.0137-12.2012. J Neurosci. 2012. PMID: 22745486 Free PMC article.
-
Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion.J Neurosci. 2013 Jan 2;33(1):175-86. doi: 10.1523/JNEUROSCI.4403-12.2013. J Neurosci. 2013. PMID: 23283332 Free PMC article.
-
Schwann cell remyelination of the central nervous system: why does it happen and what are the benefits?Open Biol. 2021 Jan;11(1):200352. doi: 10.1098/rsob.200352. Epub 2021 Jan 27. Open Biol. 2021. PMID: 33497588 Free PMC article. Review.
-
R-Ras GTPases Signaling Role in Myelin Neurodegenerative Diseases.Int J Mol Sci. 2020 Aug 17;21(16):5911. doi: 10.3390/ijms21165911. Int J Mol Sci. 2020. PMID: 32824627 Free PMC article. Review.
Cited by
-
Diverse functions of DEAD-box proteins in oligodendrocyte development, differentiation, and homeostasis.J Neurochem. 2025 Jan;169(1):e16238. doi: 10.1111/jnc.16238. Epub 2024 Oct 7. J Neurochem. 2025. PMID: 39374171 Free PMC article. Review.
-
Developmental stage-specific role of Frs adapters as mediators of FGF receptor signaling in the oligodendrocyte lineage cells.Glia. 2020 Mar;68(3):617-630. doi: 10.1002/glia.23743. Epub 2019 Oct 31. Glia. 2020. PMID: 31670856 Free PMC article.
-
Statins in Children with Neurofibromatosis Type 1: A Systematic Review of Randomized Controlled Trials.Children (Basel). 2023 Sep 15;10(9):1556. doi: 10.3390/children10091556. Children (Basel). 2023. PMID: 37761518 Free PMC article. Review.
-
Sustained ErbB Activation Causes Demyelination and Hypomyelination by Driving Necroptosis of Mature Oligodendrocytes and Apoptosis of Oligodendrocyte Precursor Cells.J Neurosci. 2021 Dec 1;41(48):9872-9890. doi: 10.1523/JNEUROSCI.2922-20.2021. Epub 2021 Nov 1. J Neurosci. 2021. PMID: 34725188 Free PMC article.
-
Neuron-oligodendroglia interactions: Activity-dependent regulation of cellular signaling.Neurosci Lett. 2020 May 14;727:134916. doi: 10.1016/j.neulet.2020.134916. Epub 2020 Mar 16. Neurosci Lett. 2020. PMID: 32194135 Free PMC article. Review.
References
-
- Bansal R, Pfeiffer SE. FGF-2 converts mature oligodendrocytes to a novel phenotype. J Neurosci Res. 1997;50:215–228. - PubMed
-
- Crawley JN. What's wrong with my mouse? In: Crawley JN, editor. Behavioral phenotype of transgenic and knockout mice. New York: Wiley; 2007. pp. 62–109.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
