Disruption of the AMPK-TBC1D1 nexus increases lipogenic gene expression and causes obesity in mice via promoting IGF1 secretion
- PMID: 27307439
- PMCID: PMC4932950
- DOI: 10.1073/pnas.1600581113
Disruption of the AMPK-TBC1D1 nexus increases lipogenic gene expression and causes obesity in mice via promoting IGF1 secretion
Abstract
Tre-2/USP6, BUB2, cdc16 domain family member 1 (the TBC domain is the GTPase activating protein domain) (TBC1D1) is a Rab GTPase activating protein that is phosphorylated on Ser(231) by the AMP-activated protein kinase (AMPK) in response to intracellular energy stress. However, the in vivo role and importance of this phosphorylation event remains unknown. To address this question, we generated a mouse model harboring a TBC1D1(Ser231Ala) knockin (KI) mutation and found that the KI mice developed obesity on a normal chow diet. Mechanistically, TBC1D1 is located on insulin-like growth factor 1 (IGF1) storage vesicles, and the KI mutation increases endocrinal and paracrinal/autocrinal IGF1 secretion in an Rab8a-dependent manner. Hypersecretion of IGF1 causes increased expression of lipogenic genes via activating the protein kinase B (PKB; also known as Akt)-mammalian target of rapamycin (mTOR) pathway in adipose tissues, which contributes to the development of obesity, diabetes, and hepatic steatosis as the KI mice age. Collectively, these findings demonstrate that the AMPK-TBC1D1 signaling nexus interacts with the PKB-mTOR pathway via IGF1 secretion, which consequently controls expression of lipogenic genes in the adipose tissue. These findings also have implications for drug discovery to combat obesity.
Keywords: AMPK; IGF1 secretion; TBC1D1; obesity; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


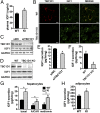

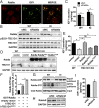
References
-
- Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13(22):2004–2008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

