High-Resolution Mapping of Ventricular Scar: Comparison Between Single and Multielectrode Catheters
- PMID: 27307518
- PMCID: PMC4911826
- DOI: 10.1161/CIRCEP.115.003841
High-Resolution Mapping of Ventricular Scar: Comparison Between Single and Multielectrode Catheters
Abstract
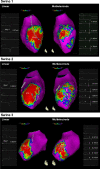
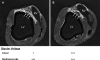
Background: Mapping resolution is influenced by electrode size and interelectrode spacing. The aims of this study were to establish normal electrogram criteria for 1-mm multielectrode-mapping catheters (Pentaray) in the ventricle and to compare its mapping resolution within scar to standard 3.5-mm catheters (Smart-Touch Thermocool).
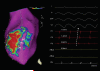
Methods and results: Three healthy swine and 11 swine with healed myocardial infarction underwent sequential mapping of the left ventricle with both catheters. Bipolar voltage amplitude in healthy tissue was similar between 3.5- and 1-mm multielectrode catheters with a 5th percentile of 1.61 and 1.48 mV, respectively. In swine with healed infarction, the total area of low bipolar voltage amplitude (defined as <1.5 mV) was 22.5% smaller using 1-mm multielectrode catheters (21.7 versus 28.0 cm2; P=0.003). This was more evident in the area of dense scar (bipolar amplitude <0.5 mV) with a 47% smaller very low-voltage area identified using 1-mm electrode catheters (7.1 versus 15.2 cm(2); P=0.003). In this region, 1-mm multielectrode catheters recorded higher voltage amplitude (0.72±0.81 mV versus 0.30±0.12 mV; P<0.001). Importantly, 27% of these dense scar electrograms showed distinct triphasic electrograms when mapped using a 1-mm multielectrode catheter compared with fractionated multicomponent electrogram recorded with the 3.5-mm electrode catheter. In 8 mapped reentrant ventricular tachycardias, the circuits included regions of preserved myocardial tissue channels identified with 1-mm multielectrode catheters but not 3.5-mm electrode catheters. Pacing threshold within the area of low voltage was lower with 1-mm electrode catheters (0.9±1.3 mV versus 3.8±3.7 mV; P=0.001).
Conclusions: Mapping with small closely spaced electrode catheters can improve mapping resolution within areas of low voltage.
Keywords: electrodes; heart; myocardial infarction; swine; ventricular tachycardia.
© 2016 American Heart Association, Inc.
Figures





Comment in
-
Emergence of Multielectrode Mapping: On the Road to Higher Resolution.Circ Arrhythm Electrophysiol. 2016 Jun;9(6):e004281. doi: 10.1161/CIRCEP.116.004281. Circ Arrhythm Electrophysiol. 2016. PMID: 27307521 No abstract available.
References
-
- Fenoglio JJ, Jr., Pham TD, Harken AH, Horowitz LN, Josephson ME, Wit AL. Recurrent sustained ventricular tachycardia: structure and ultrastructure of subendocardial regions in which tachycardia originates. Circulation. 1983;68:518–533. - PubMed
-
- de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, Dingemans KP, van Hemel NM, Hauer RN. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606. - PubMed
-
- Anter E, Tschabrunn CM, Buxton AEJM. Characteristics of Reentrant Ventricular Tachycardia in Healed Myocardial Infarction. Heart Rhythm. 2015;12:S204.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

