Root cap-dependent gravitropic U-turn of maize root requires light-induced auxin biosynthesis via the YUC pathway in the root apex
- PMID: 27307546
- PMCID: PMC4973731
- DOI: 10.1093/jxb/erw232
Root cap-dependent gravitropic U-turn of maize root requires light-induced auxin biosynthesis via the YUC pathway in the root apex
Abstract
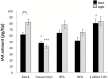
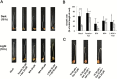
Gravitropism refers to the growth or movement of plants that is influenced by gravity. Roots exhibit positive gravitropism, and the root cap is thought to be the gravity-sensing site. In some plants, the root cap requires light irradiation for positive gravitropic responses. However, the mechanisms regulating this phenomenon are unknown. We herein report that maize roots exposed to white light continuously for ≥1-2h show increased indole-3-acetic acid (IAA) levels in the root tips, especially in the transition zone (1-3mm from the tip). Treatment with IAA biosynthesis inhibitors yucasin and l-kynurenine prevented any increases in IAA content and root curvature under light conditions. Analyses of the incorporation of a stable isotope label from tryptophan into IAA revealed that some of the IAA in roots was synthesized in the root apex. Furthermore, Zmvt2 and Zmyuc gene transcripts were detected in the root apex. One of the Zmyuc genes (ZM2G141383) was up-regulated by light irradiation in the 0-1mm tip region. Our findings suggest that IAA accumulation in the transition zone is due to light-induced activation of Zmyuc gene expression in the 0-1mm root apex region. Light-induced changes in IAA levels and distributions mediate the maize root gravitropic U-turn.
Keywords: Indole-3-acetic acid (IAA) biosynthesis; Zmvt2; Zmyuc.; light dependent; maize; root cap; root gravitropism; yucasin.
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures





Similar articles
-
Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis.Plant J. 2014 Feb;77(3):352-66. doi: 10.1111/tpj.12399. Epub 2014 Jan 16. Plant J. 2014. PMID: 24299123
-
Effects of abscisic acid and xanthoxin on elongation and gravitropism in primary roots of Zea mays.Plant Sci. 1990;68:17-26. doi: 10.1016/0168-9452(90)90147-g. Plant Sci. 1990. PMID: 11538694
-
Patterns of auxin and abscisic acid movement in the tips of gravistimulated primary roots of maize.Plant Growth Regul. 1996;20:253-8. doi: 10.1007/BF00043315. Plant Growth Regul. 1996. PMID: 11540494
-
How roots perceive and respond to gravity.Am J Bot. 1986 Apr;73(4):574-87. Am J Bot. 1986. PMID: 11540848 Review.
-
Differential growth and hormone redistribution in gravireacting maize roots.Environ Exp Bot. 1989 Jan;29(1):37-45. doi: 10.1016/0098-8472(89)90037-3. Environ Exp Bot. 1989. PMID: 11541034 Review.
Cited by
-
Primary Root and Mesocotyl Elongation in Maize Seedlings: Two Organs with Antagonistic Growth below the Soil Surface.Plants (Basel). 2021 Jun 23;10(7):1274. doi: 10.3390/plants10071274. Plants (Basel). 2021. PMID: 34201525 Free PMC article. Review.
-
Light Signaling, Root Development, and Plasticity.Plant Physiol. 2018 Feb;176(2):1049-1060. doi: 10.1104/pp.17.01079. Epub 2017 Sep 22. Plant Physiol. 2018. PMID: 28939624 Free PMC article. Review.
-
Phytochrome and Phytohormones: Working in Tandem for Plant Growth and Development.Front Plant Sci. 2018 Jul 27;9:1037. doi: 10.3389/fpls.2018.01037. eCollection 2018. Front Plant Sci. 2018. PMID: 30100912 Free PMC article. Review.
-
Gravity Reduced Nitrogen Uptake via the Regulation of Brace Unilateral Root Growth in Maize Intercropping.Front Plant Sci. 2021 Sep 6;12:724909. doi: 10.3389/fpls.2021.724909. eCollection 2021. Front Plant Sci. 2021. PMID: 34552608 Free PMC article.
-
Comparative Transcriptome Analysis Reveal Candidate Genes Potentially Involved in Regulation of Primocane Apex Rooting in Raspberry (Rubus spp.).Front Plant Sci. 2017 Jun 13;8:1036. doi: 10.3389/fpls.2017.01036. eCollection 2017. Front Plant Sci. 2017. PMID: 28659963 Free PMC article.
References
-
- Berleth T, Sachs T. 2001. Plant morphogenesis: long-distance coordination and local patterning. Current Opinion in Plant Biology 4, 57–62. - PubMed
-
- Brunoud G, Wells DM, Oliva M, et al. 2012. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482, 103–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

