Bacterial Sphingomyelinases and Phospholipases as Virulence Factors
- PMID: 27307578
- PMCID: PMC4981679
- DOI: 10.1128/MMBR.00082-15
Bacterial Sphingomyelinases and Phospholipases as Virulence Factors
Abstract
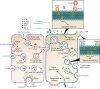
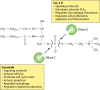
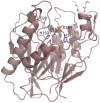
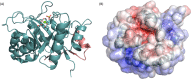
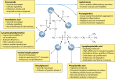
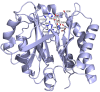
Bacterial sphingomyelinases and phospholipases are a heterogeneous group of esterases which are usually surface associated or secreted by a wide variety of Gram-positive and Gram-negative bacteria. These enzymes hydrolyze sphingomyelin and glycerophospholipids, respectively, generating products identical to the ones produced by eukaryotic enzymes which play crucial roles in distinct physiological processes, including membrane dynamics, cellular signaling, migration, growth, and death. Several bacterial sphingomyelinases and phospholipases are essential for virulence of extracellular, facultative, or obligate intracellular pathogens, as these enzymes contribute to phagosomal escape or phagosomal maturation avoidance, favoring tissue colonization, infection establishment and progression, or immune response evasion. This work presents a classification proposal for bacterial sphingomyelinases and phospholipases that considers not only their enzymatic activities but also their structural aspects. An overview of the main physiopathological activities is provided for each enzyme type, as are examples in which inactivation of a sphingomyelinase- or a phospholipase-encoding gene impairs the virulence of a pathogen. The identification of sphingomyelinases and phospholipases important for bacterial pathogenesis and the development of inhibitors for these enzymes could generate candidate vaccines and therapeutic agents, which will diminish the impacts of the associated human and animal diseases.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
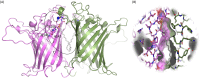

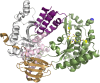







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

