Virion Glycoprotein-Mediated Immune Evasion by Human Cytomegalovirus: a Sticky Virus Makes a Slick Getaway
- PMID: 27307580
- PMCID: PMC4981668
- DOI: 10.1128/MMBR.00018-16
Virion Glycoprotein-Mediated Immune Evasion by Human Cytomegalovirus: a Sticky Virus Makes a Slick Getaway
Abstract
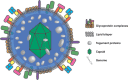
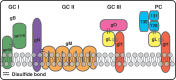
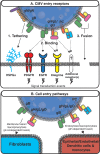
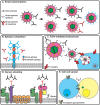
The prototypic herpesvirus human cytomegalovirus (CMV) exhibits the extraordinary ability to establish latency and maintain a chronic infection throughout the life of its human host. This is even more remarkable considering the robust adaptive immune response elicited by infection and reactivation from latency. In addition to the ability of CMV to exist in a quiescent latent state, its persistence is enabled by a large repertoire of viral proteins that subvert immune defense mechanisms, such as NK cell activation and major histocompatibility complex antigen presentation, within the cell. However, dissemination outside the cell presents a unique existential challenge to the CMV virion, which is studded with antigenic glycoprotein complexes targeted by a potent neutralizing antibody response. The CMV virion envelope proteins, which are critical mediators of cell attachment and entry, possess various characteristics that can mitigate the humoral immune response and prevent viral clearance. Here we review the CMV glycoprotein complexes crucial for cell attachment and entry and propose inherent properties of these proteins involved in evading the CMV humoral immune response. These include viral glycoprotein polymorphism, epitope competition, Fc receptor-mediated endocytosis, glycan shielding, and cell-to-cell spread. The consequences of CMV virion glycoprotein-mediated immune evasion have a major impact on persistence of the virus in the population, and a comprehensive understanding of these evasion strategies will assist in designing effective CMV biologics and vaccines to limit CMV-associated disease.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

