Sorafenib and Quinacrine Target Anti-Apoptotic Protein MCL1: A Poor Prognostic Marker in Anaplastic Thyroid Cancer (ATC)
- PMID: 27307592
- PMCID: PMC7524204
- DOI: 10.1158/1078-0432.CCR-15-2792
Sorafenib and Quinacrine Target Anti-Apoptotic Protein MCL1: A Poor Prognostic Marker in Anaplastic Thyroid Cancer (ATC)
Abstract
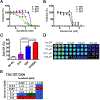
Purpose and experimental design: Anaplastic thyroid cancer (ATC) comprises approximately 2% of all thyroid cancers, and its median survival rate remains poor. It is responsible for more than one third of thyroid cancer-related deaths. ATC is frequently resistant to conventional therapy, and NFκB signaling has been proposed to be a feature of the disease. We aimed to assess the activity of the antimalaria drug quinacrine known to target NFκB signaling in combination with the clinically relevant kinase inhibitor sorafenib in ATC cells. The presence of NFκB-p65/RELA and its target MCL1 was demonstrated in ATC by meta-data gene set enrichment analysis and IHC. We assessed the responses of a panel of human ATC cell lines to quinacrine and sorafenib in vitro and in vivo RESULTS: We detected increased expression of NFκB-p65/RELA and MCL1 in the nucleus of a subset of ATC compared with non-neoplastic thyroid. ATC cells were found to respond with additive/synergistic tumor cell killing to the combination of sorafenib plus quinacrine in vitro, and the drug combination improves survival of immunodeficient mice injected orthotopically with ATC cells as compared with mice administered either compound alone or doxorubicin. We also demonstrate that the combination of sorafenib and quinacrine is well tolerated in mice. At the molecular level, quinacrine and sorafenib inhibited expression of prosurvival MCL1, pSTAT3, and dampened NFκB signaling.
Conclusions: The combination of quinacrine and sorafenib targets emerging molecular hallmarks of ATC and shows promising results in clinically relevant models for the disease. Further testing of sorafenib plus quinacrine can be conducted in ATC patients. Clin Cancer Res; 22(24); 6192-203. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures

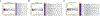
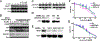
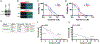
References
-
- Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer control : journal of the Moffitt Cancer Center. 2006;13:119–28. - PubMed
-
- Woyach JA, Shah MH. New therapeutic advances in the management of progressive thyroid cancer. Endocrine-related cancer. 2009;16:715–31. - PubMed
-
- Wu H, Sun Y, Ye H, Yang S, Lee SL, de Las Morenas A. Anaplastic Thyroid Cancer: Outcome and the Mutation/Expression Profiles of Potential Targets. Pathology oncology research : POR. 2015. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

