Comparative evaluation of dexmedetomidine and midazolam-ketamine combination as sedative agents in pediatric dentistry: A double-blinded randomized controlled trial
- PMID: 27307665
- PMCID: PMC4906861
- DOI: 10.4103/0976-237X.183058
Comparative evaluation of dexmedetomidine and midazolam-ketamine combination as sedative agents in pediatric dentistry: A double-blinded randomized controlled trial
Abstract
Background: Pharmacological methods have been used as an adjunct to enhance child cooperativeness and facilitate dental treatment.
Objective: Purpose of this study was to evaluate and compare the effect of sedation by intranasal dexmedetomidine and oral combination drug midazolam-ketamine in a group of children with uncooperative behavior requiring dental treatment.
Materials and methods: This was a prospective, randomized, double-blind study that included patients 3-9 years old with American Society of Anesthesiologists-I status. About 36 children presenting early childhood caries were randomly assigned to one of three groups studied: Group MK received intranasal saline and oral midazolam (0.5 mg/kg) with ketamine (5 mg/kg) mixed in mango juice; Group DX received intranasal dexmedetomidine (1 μg/kg) and oral mango juice; and Group C received intranasal saline and oral mango juice. Patients' heart rate, blood pressure, and oxygen saturation were recorded before, during, and at the end of the procedure. Patients' behavior, sedation status, and wake up behavior were evaluated with modified observer assessment of alertness and sedation scale. Ease of treatment completion was evaluated according to Houpt scale.
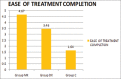
Results: Hemodynamic changes were statistically insignificant in Group MK and Group DX. About 75% patients in Group MK were successfully sedated as compared to 53.9% Group DX and none of the patients in Group C. Ease of treatment completion was better with Group MK as compared to Group DX and least with Group C. Around 50% patients in Group MK had postoperative complications.
Conclusion: Oral midazolam-ketamine combination and intranasal dexmedetomidine evaluated in the present study can be used safely and effectively in uncooperative pediatric dental patients for producing conscious sedation.
Keywords: Dexmedetomidine; midazolam–ketamine; pediatric dentistry; sedation.
Figures
References
-
- Surendar MN, Pandey RK, Saksena AK, Kumar R, Chandra G. A comparative evaluation of intranasal dexmedetomidine, midazolam and ketamine for their sedative and analgesic properties: A triple blind randomized study. J Clin Pediatr Dent. 2014;38:255–61. - PubMed
-
- Raadal M, Lundeberg S, Haukali G. Pain, pain control and sedation. In: Koch G, Poulsen S, editors. Pediatric Dentistry, A Clinical Approach. 2nd ed. Singapore: Blackwell; 2009. p. 54.
-
- Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov Today. 2002;7:967–75. - PubMed
-
- Kanto JH. Midazolam: The first water-soluble benzodiazepine. Pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Pharmacotherapy. 1985;5:138–55. - PubMed
-
- Kain ZN, Mayes LC, Bell C, Weisman S, Hofstadter MB, Rimar S. Premedication in the United States: A status report. Anesth Analg. 1997;84:427–32. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical