Polarity-sensitive nanocarrier for oral delivery of Sb(V) and treatment of cutaneous leishmaniasis
- PMID: 27307731
- PMCID: PMC4887043
- DOI: 10.2147/IJN.S105952
Polarity-sensitive nanocarrier for oral delivery of Sb(V) and treatment of cutaneous leishmaniasis
Abstract
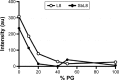
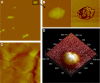
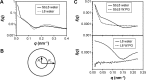
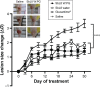
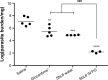
There is a great need for orally active drugs for the treatment of the neglected tropical disease leishmaniasis. Amphiphilic Sb(V) complexes, such as 1:3 Sb-N-octanoyl-N-methylglucamide complex (SbL8), are promising drug candidates. It has been previously reported that SbL8 forms kinetically stabilized nanoassemblies in water and that this simple dispersion exhibits antileishmanial activity when given by oral route to a murine model of visceral leishmaniasis. The main objective of the present work was to interfere in the structural organization of these nanoassemblies so as to investigate their influence on the oral bioavailability of Sb, and ultimately, optimize an oral formulation of SbL8 for the treatment of cutaneous leishmaniasis. The structural organization of SbL8 nanoassemblies was manipulated through addition of propylene glycol (PG) to the aqueous dispersion of SbL8. The presence of 50% (v/v) PG resulted in the loss of hydrophobic microenvironment, as evidenced by fluorescence probing. However, nanostructures were still present, as demonstrated by dynamic light scattering, small-angle X-ray scattering, and atomic force microscopy (AFM). A remarkable property of these nanoassemblies, as revealed by AFM analysis, is the flexibility of their supramolecular organization, which showed changes as a function of the solvent and substrate polarities. The formulation of SbL8 in 1:1 water:PG given orally to mice promoted significantly higher and more sustained serum levels of Sb, when compared to SbL8 in water. The new formulation, when given as repeated doses (200 mg Sb/kg/day) to BALB/c mice infected with Leishmania amazonensis, was significantly more effective in reducing the lesion parasite burden, compared to SbL8 in water, and even, the conventional drug Glucantime(®) given intraperitoneally at the same dose. In conclusion, this work introduces a new concept of polarity-sensitive nanocarrier that was successfully applied to optimize an oral formulation of Sb(V) for treating cutaneous leishmaniasis.
Keywords: AFM; Leishmania amazonensis; SAXS; amphiphilic complex; antimony; propylene glycol.
Figures



References
-
- Marsden PD. Pentavalent antimonials: old drugs for new diseases. Rev Soc Bras Med Trop. 1985;18:187–198.
-
- Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. - PubMed
-
- Ridley RG. The need for new approaches to tropical disease drug discovery and development for improved control strategies. In: Failamb AH, Ridley RG, Vial HJ, editors. Drugs Against Parasitic Diseases: R&D Methodologies and Issues. Geneva: UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR); 2003. pp. 13–21.
-
- Organización Panamericana de la Salud . Leishmaniasis en las Américas: recomendaciones para el tratamiento [Leishmaniasis in the Americas: Recommendations for Treatment] Washington, DC: OPS; 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

