Organocatalytic Synthesis of Higher-Carbon Sugars: Efficient Protocol for the Synthesis of Natural Sedoheptulose and d-Glycero-l-galacto-oct-2-ulose
- PMID: 27308197
- PMCID: PMC4906512
- DOI: 10.1002/open.201500099
Organocatalytic Synthesis of Higher-Carbon Sugars: Efficient Protocol for the Synthesis of Natural Sedoheptulose and d-Glycero-l-galacto-oct-2-ulose
Abstract
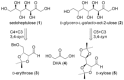
Herein we report a short and efficient protocol for the synthesis of naturally occurring higher-carbon sugars-sedoheptulose (d-altro-hept-2-ulose) and d-glycero-l-galacto-oct-2-ulose-from readily available sugar aldehydes and dihydroxyacetone (DHA). The key step includes a diastereoselective organocatalytic syn-selective aldol reaction of DHA with d-erythrose and d-xylose, respectively. The methodology presented can be expanded to the synthesis of various higher sugars by means of syn-selective carbon-carbon-bond-forming aldol reactions promoted by primary-based organocatalysts. For example, this methodology provided useful access to d-glycero-d-galacto-oct-2-ulose and 1-deoxy-d-glycero-d-galacto-oct-2-ulose from d-arabinose in high yield (85 and 74 %, respectively) and high stereoselectivity (99:1).
Keywords: aldol reaction; asymmetric synthesis; carbohydrates; natural products; organocatalysis.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

