Epigenetic control of gene expression in leukemogenesis: Cooperation between wild type MLL and MLL fusion proteins
- PMID: 27308325
- PMCID: PMC4905190
- DOI: 10.1080/23723548.2014.955330
Epigenetic control of gene expression in leukemogenesis: Cooperation between wild type MLL and MLL fusion proteins
Abstract
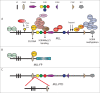
Although there has been great progress in the treatment of human cancers, especially leukemias, many remain resistant to treatment. A major current focus is the development of so-called epigenetic drugs. Epigenetic states are stable enough to persist through multiple cell divisions, but by their very nature are reversible and thus are amenable to therapeutic manipulation. Exciting work in this area has produced a new breed of highly specific small molecules designed to inhibit epigenetic proteins, some of which have entered clinical trials. The current and future development of epigenetic drugs is greatly aided by highly detailed information about normal and aberrant epigenetic changes at the molecular level. In this review we focus on a class of aggressive acute leukemias caused by mutations in the Mixed Lineage Leukemia (MLL) gene. We provide an overview of how detailed molecular analysis of MLL leukemias has provided several early-stage epigenetic drugs and propose that further study of MLL leukemogenesis may continue to provide molecular details that potentially have a wider range of applications in human cancers.
Keywords: MLL; acetylation; chromatin modifications; epigenetic; fusion proteins; histone; leukemia; methylation; small molecule inhibitors; therapy; transcription elongation.
Figures

References
-
- Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat Rev Cancer 2013; 13:497-510; PMID:23760024; http://dx.doi.org/10.1038/nrc3486 - DOI - PMC - PubMed
-
- Luger K, Dechassa ML, Tremethick DJ. New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nat Rev Mol Cell Biol 2012; 13:436-47; PMID:22722606; http://dx.doi.org/10.1038/nrm3382 - DOI - PMC - PubMed
-
- Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet 2011; 12:7-18; PMID:21116306; http://dx.doi.org/10.1038/nrg2905 - DOI - PubMed
-
- Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell 2012; 150:12-27; PMID:22770212; http://dx.doi.org/10.1016/j.cell.2012.06.013 - DOI - PubMed
-
- Greaves M, Maley CC. Clonal evolution in cancer. Nature 2012; 481:306-13; PMID:22258609; http://dx.doi.org/10.1038/nature10762 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
