Efficacy and Safety of Combined Androgen Deprivation Therapy (ADT) and Docetaxel Compared with ADT Alone for Metastatic Hormone-Naive Prostate Cancer: A Systematic Review and Meta-Analysis
- PMID: 27308831
- PMCID: PMC4911003
- DOI: 10.1371/journal.pone.0157660
Efficacy and Safety of Combined Androgen Deprivation Therapy (ADT) and Docetaxel Compared with ADT Alone for Metastatic Hormone-Naive Prostate Cancer: A Systematic Review and Meta-Analysis
Abstract
Objective: Prostate cancer is the most common nonskin cancer and second most common cause of cancer mortality in older men in the United States (USA) and Western Europe. Androgen-deprivation therapy alone (ADT) remains the first line of treatment in most cases, for metastatic disease. We performed a systematic review and meta-analysis of all randomized controlled trials (RCT) that compared the efficacy and adverse events profile of a chemohormonal therapy (ADT ± docetaxel) for metastatic hormone-naive prostate cancer (mHNPC).
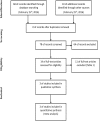
Methods: Several databases were searched, including MEDLINE, EMBASE, LILACS, and CENTRAL. The primary endpoint was overall survival. Data extracted from the studies were combined by using the hazard ratio (HR) or risk ratio (RR) with their corresponding 95% confidence intervals (95% CI).
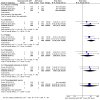
Results: The final analysis included 3 trials comprising 2,264 patients (mHNPC). Patients who received the chemohormonal therapy had a longer clinical progression-free survival interval (HR = 0.64; 95% CI: 0.55 to 0.75; p<0.00001), and no heterogeneity (Chi2 = 0.64; df = 1 [p = 0.42]; I2 = 0%). The biochemical progression-free survival (bPFS) also was higher in patients treated with ADT plus docetaxel (HR = 0.63; 95% CI: 0.57 to 0.69; p<0.00001), also with no heterogeneity noted (Chi2 = 0.48; df = 2 [p = 0.79]; I2 = 0%). Finally, the combination of ADT with docetaxel showed a superior overall survival (OS) compared with ADT alone (HR = 0.73; 95% CI: 0.64 to 0.84; p<0.0001), with moderate heterogeneity (Chi2 = 3.84; df = 2 [p = 0.15]; I2 = 48%). A random-effects model analysis was performed, and the results remained favorable to the use of ADT plus docetaxel (HR = 0.73; 95% CI: 0.60 to 0.89; p = 0.002). In the final combined analysis of the high-volume disease patients, the use of the combination therapy also favored an increased overall survival (HR = 0.67; 95% CI: 0.54 to 0.83; p = 0.0003). Regarding adverse events and severe toxicity (grade ≥3), the group receiving the combined therapy had higher rates of neutropenia, febrile neutropenia and fatigue.
Conclusion: The combination of ADT with docetaxel improved the clinical progression-free survival, bPFS and OS of patients with mHNPC. A superior OS was seen especially for patients with metastatic and high-volume disease. This contemporary combination therapy may now be offered as a first-line treatment for selected patients.
Conflict of interest statement
Figures

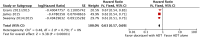
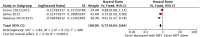
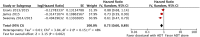
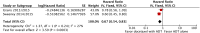
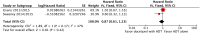

References
-
- Botrel TE, Clark O, Pompeo AC, Bretas FF, Sadi MV, Ferreira U, et al. Immunotherapy with Sipuleucel-T (APC8015) in patients with metastatic castration-refractory prostate cancer (mCRPC): a systematic review and meta-analysis. International braz j urol: official journal of the Brazilian Society of Urology. 2012;38(6):717–27. Epub 2013/01/11. . - PubMed
-
- Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25(12):1596–605. Epub 2007/04/04. doi: JCO.2006.10.1949 [pii] 10.1200/JCO.2006.10.1949 . - DOI - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. European urology. 2014;65(2):467–79. Epub 2013/12/11. 10.1016/j.eururo.2013.11.002 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

