Detection of Avian H7N9 Influenza A Viruses in the Yangtze Delta Region of China During Early H7N9 Outbreaks
- PMID: 27309047
- PMCID: PMC4911810
- DOI: 10.1637/11098-042015-Reg
Detection of Avian H7N9 Influenza A Viruses in the Yangtze Delta Region of China During Early H7N9 Outbreaks
Abstract
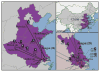
Since the first H7N9 human case in Shanghai, February 19, 2013, the emerging avian-origin H7N9 influenza A virus has become an epizootic virus in China, posing a potential pandemic threat to public health. From April 2 to April 28, 2013, some 422 oral-pharyngeal and cloacal swabs were collected from birds and environmental surfaces at five live poultry markets (LPMs) and 13 backyard poultry farms (BPFs) across three cities, Wuxi, Suzhou, and Nanjing, in the Yangtze Delta region. In total 22 isolates were recovered, and six were subtyped as H7N9, nine as H9N2, four as H7N9/H9N2, and three unsubtyped influenza A viruses. Genomic sequences showed that the HA and NA genes of the H7N9 viruses were similar to those of the H7N9 human isolates, as well as other avian-origin H7N9 isolates in the region, but the PB1, PA, NP, and MP genes of the sequenced viruses were more diverse. Among the four H7N9/H9N2 mixed infections, three were from LPM, whereas the other one was from the ducks at one BPF, which were H7N9 negative in serologic analyses. A survey of the bird trading records of the LPMs and BPFs indicates that trading was a likely route for virus transmission across these regions. Our results suggested that better biosecurity and more effective vaccination should be implemented in backyard farms, in addition to biosecurity management in LPMs.
Keywords: H7N9; avian influenza virus; backyard poultry farm; domestic poultry; influenza A virus; live poultry market; wild bird.
Figures


References
-
- Campitelli L, Mogavero E, De Marco MA, Delogu M, Puzelli S, Frezza F, Facchini M, Chiapponi C, Foni E, Cordioli P, Webby R, Barigazzi G, Webster RG, Donatelli I. Interspecies transmission of an H7N3 influenza virus from wild birds to intensively reared domestic poultry in Italy. Virology. 2004;323:24–36. - PubMed
-
- Edwards S. OIE laboratory standards for avian influenza. Developments in biologicals. 2006;124:159–162. - PubMed
-
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. - PubMed
-
- Gilbert M, Golding N, Zhou H, Wint GR, Robinson TP, Tatem AJ, Lai S, Zhou S, Jiang H, Guo D, Huang Z, Messina JP, Xiao X, Linard C, Van Boeckel TP, Martin V, Bhatt S, Gething PW, Farrar JJ, Hay SI, Yu H. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat Commun. 2014;5:4116. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

