Photofunctionalization of Titanium: An Alternative Explanation of Its Chemical-Physical Mechanism
- PMID: 27309723
- PMCID: PMC4911147
- DOI: 10.1371/journal.pone.0157481
Photofunctionalization of Titanium: An Alternative Explanation of Its Chemical-Physical Mechanism
Abstract
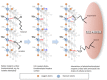
Objectives: To demonstrate that titanium implant surfaces as little as 4 weeks from production are contaminated by atmospheric hydrocarbons. This phenomenon, also known as biological ageing can be reversed by UVC irradiation technically known as photofunctionalization. To propose a new model from our experimental evidence to explain how the changes in chemical structure of the surface will affect the adsorption of amino acids on the titanium surface enhancing osteointegration.
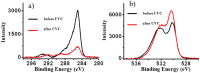
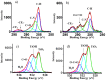
Methods: In our study XPS and AES were used to analyze the effects of UVC irradiation (photofunctionalization) in reversing biological ageing of titanium. SEM was used to analyze any possible effects on the topography of the surface.
Results: UVC irradiation was able to reverse biological ageing of titanium by greatly reducing the amount of carbon contamination present on the implant surface by up to 4 times, while the topography of the surface was not affected. UVC photon energy reduces surface H2O and increases TiOH with many -OH groups being produced. These groups explain the super-hydrophilic effect from photofunctionalization when these groups come into contact with water.
Significance: Photofunctionalization has proven to be a valid method to reduce the amount of hydrocarbon contamination on titanium dental implants and improve biological results. The chemisorption mechanisms of amino acids, in our study, are dictated by the chemical structure and electric state present on the surface, but only in the presence of an also favourable geometrical composition at the atomical level.
Conflict of interest statement
Figures



References
-
- Ratner BD, Allan SH, Schoen FJ, Lemons JE. An introduction to materials in Medicine. Biomaterials Science, Academic Press; 1996.
-
- Li C, Monti S, Carravetta V. Journey toward the Surface: How Glycine Adsorbs on Titania in Water Solution. The Journal of Physical Chemistry C 2012;116:18318–26.
-
- Steinemann SG. Titanium—the material of choice? Periodontology 2000 1998;17:7–21. - PubMed
-
- Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dental materials: official publication of the Academy of Dental Materials 2007;23:844–54. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

