Remyelination Is Correlated with Regulatory T Cell Induction Following Human Embryoid Body-Derived Neural Precursor Cell Transplantation in a Viral Model of Multiple Sclerosis
- PMID: 27310015
- PMCID: PMC4911106
- DOI: 10.1371/journal.pone.0157620
Remyelination Is Correlated with Regulatory T Cell Induction Following Human Embryoid Body-Derived Neural Precursor Cell Transplantation in a Viral Model of Multiple Sclerosis
Abstract
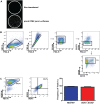
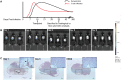
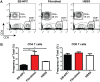
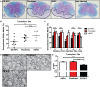
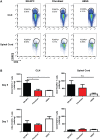
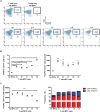
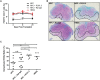
We have recently described sustained clinical recovery associated with dampened neuroinflammation and remyelination following transplantation of neural precursor cells (NPCs) derived from human embryonic stem cells (hESCs) in a viral model of the human demyelinating disease multiple sclerosis. The hNPCs used in that study were derived by a novel direct differentiation method (direct differentiation, DD-NPCs) that resulted in a unique gene expression pattern when compared to hNPCs derived by conventional methods. Since the therapeutic potential of human NPCs may differ greatly depending on the method of derivation and culture, we wanted to determine whether NPCs differentiated using conventional methods would be similarly effective in improving clinical outcome under neuroinflammatory demyelinating conditions. For the current study, we utilized hNPCs differentiated from a human induced pluripotent cell line via an embryoid body intermediate stage (EB-NPCs). Intraspinal transplantation of EB-NPCs into mice infected with the neurotropic JHM strain of mouse hepatitis virus (JHMV) resulted in decreased accumulation of CD4+ T cells in the central nervous system that was concomitant with reduced demyelination at the site of injection. Dampened neuroinflammation and remyelination was correlated with a transient increase in CD4+FOXP3+ regulatory T cells (Tregs) concentrated within the peripheral lymphatics. However, compared to our earlier study, pathological improvements were modest and did not result in significant clinical recovery. We conclude that the genetic signature of NPCs is critical to their effectiveness in this model of viral-induced neurologic disease. These comparisons will be useful for understanding what factors are critical for the sustained clinical improvement.
Conflict of interest statement
Figures

Similar articles
-
Promoting remyelination through cell transplantation therapies in a model of viral-induced neurodegenerative disease.Dev Dyn. 2019 Jan;248(1):43-52. doi: 10.1002/dvdy.24658. Epub 2018 Sep 6. Dev Dyn. 2019. PMID: 30067309 Free PMC article. Review.
-
MHC mismatch results in neural progenitor cell rejection following spinal cord transplantation in a model of viral-induced demyelination.Stem Cells. 2012 Nov;30(11):2584-95. doi: 10.1002/stem.1234. Stem Cells. 2012. PMID: 22969049 Free PMC article.
-
Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant.Neurobiol Dis. 2020 Jul;140:104868. doi: 10.1016/j.nbd.2020.104868. Epub 2020 Apr 8. Neurobiol Dis. 2020. PMID: 32276110 Free PMC article.
-
Two-photon imaging of remyelination of spinal cord axons by engrafted neural precursor cells in a viral model of multiple sclerosis.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):E2349-55. doi: 10.1073/pnas.1406658111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843159 Free PMC article.
-
Promoting remyelination: utilizing a viral model of demyelination to assess cell-based therapies.Expert Rev Neurother. 2014 Oct;14(10):1169-79. doi: 10.1586/14737175.2014.955854. Expert Rev Neurother. 2014. PMID: 25245576 Free PMC article. Review.
Cited by
-
Brain regulatory T cells.Nat Rev Immunol. 2024 May;24(5):326-337. doi: 10.1038/s41577-023-00960-z. Epub 2023 Dec 1. Nat Rev Immunol. 2024. PMID: 38040953 Review.
-
Regulatory T cell therapy for multiple sclerosis: Breaching (blood-brain) barriers.Hum Vaccin Immunother. 2022 Dec 30;18(7):2153534. doi: 10.1080/21645515.2022.2153534. Epub 2022 Dec 28. Hum Vaccin Immunother. 2022. PMID: 36576251 Free PMC article. Review.
-
Stem Cells as Potential Targets of Polyphenols in Multiple Sclerosis and Alzheimer's Disease.Biomed Res Int. 2018 Jul 12;2018:1483791. doi: 10.1155/2018/1483791. eCollection 2018. Biomed Res Int. 2018. PMID: 30112360 Free PMC article. Review.
-
OCA-B promotes pathogenic maturation of stem-like CD4+ T cells and autoimmune demyelination.J Clin Invest. 2025 Apr 29;135(13):e187862. doi: 10.1172/JCI187862. eCollection 2025 Jul 1. J Clin Invest. 2025. PMID: 40299553 Free PMC article.
-
Promoting remyelination through cell transplantation therapies in a model of viral-induced neurodegenerative disease.Dev Dyn. 2019 Jan;248(1):43-52. doi: 10.1002/dvdy.24658. Epub 2018 Sep 6. Dev Dyn. 2019. PMID: 30067309 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

