Formal Models of the Network Co-occurrence Underlying Mental Operations
- PMID: 27310288
- PMCID: PMC4911040
- DOI: 10.1371/journal.pcbi.1004994
Formal Models of the Network Co-occurrence Underlying Mental Operations
Abstract
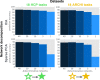
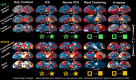
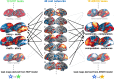
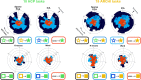
Systems neuroscience has identified a set of canonical large-scale networks in humans. These have predominantly been characterized by resting-state analyses of the task-unconstrained, mind-wandering brain. Their explicit relationship to defined task performance is largely unknown and remains challenging. The present work contributes a multivariate statistical learning approach that can extract the major brain networks and quantify their configuration during various psychological tasks. The method is validated in two extensive datasets (n = 500 and n = 81) by model-based generation of synthetic activity maps from recombination of shared network topographies. To study a use case, we formally revisited the poorly understood difference between neural activity underlying idling versus goal-directed behavior. We demonstrate that task-specific neural activity patterns can be explained by plausible combinations of resting-state networks. The possibility of decomposing a mental task into the relative contributions of major brain networks, the "network co-occurrence architecture" of a given task, opens an alternative access to the neural substrates of human cognition.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

