Pharmacokinetics, Safety, and Tolerability of Fevipiprant (QAW039), a Novel CRTh2 Receptor Antagonist: Results From 2 Randomized, Phase 1, Placebo-Controlled Studies in Healthy Volunteers
- PMID: 27310331
- PMCID: PMC5071756
- DOI: 10.1002/cpdd.244
Pharmacokinetics, Safety, and Tolerability of Fevipiprant (QAW039), a Novel CRTh2 Receptor Antagonist: Results From 2 Randomized, Phase 1, Placebo-Controlled Studies in Healthy Volunteers
Erratum in
-
Correction.Clin Pharmacol Drug Dev. 2020 Oct;9(7):889-890. doi: 10.1002/cpdd.861. Epub 2020 Aug 9. Clin Pharmacol Drug Dev. 2020. PMID: 33038292 Free PMC article. No abstract available.
Abstract
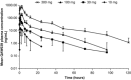
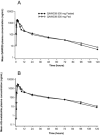
We evaluated the pharmacokinetics (PK), safety, and tolerability of a novel oral CRTh2 antagonist, fevipiprant (QAW039), in healthy subjects. Peak concentrations of fevipiprant in plasma were observed 1-3 hours postdosing. Concentrations declined in a multiexponential manner, followed by an apparent terminal phase (t1/2 , ∼20 hours). Steady state was achieved in 4 days with <2-fold accumulation. Elimination was partly by renal excretion (≤30% of the dose) and glucuronidation. Food had minimal impact on the PK of fevipiprant, and it was well tolerated at single and multiple oral doses up to 500 mg/day. No dose-dependent adverse events were observed, and all the events were mild or moderate in severity. Systemic concentrations were sufficiently high to achieve relevant target occupancy, considering in vitro pharmacology data. In summary, the data support further development as a once-daily oral therapy for allergic diseases.
Keywords: QAW039; fevipiprant; healthy subjects; pharmacokinetics; safety.
© 2016, The Authors. Clinical Pharmacology in Drug Development Published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Figures
References
-
- Lewis RA, Soter NA, Diamond PT, Austen KF, JA Oates, Roberts LJ 2nd. Prostaglandin D2 generation after activation of rat and human mast cells with anti‐IgE. J Immunol. 1982;129:1627‒1631. - PubMed
-
- Murray JJ, Tonnel AB, Brash AR, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986;315:800‒804. - PubMed
-
- Doyle WJ, Boehm S, Skoner DP. Physiologic responses to intranasal dose‐response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J Allergy Clin Immunol. 1990;86:924‒935. - PubMed
-
- Kostenis E, Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol Med. 2006;12:148‒158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources