Boiling and Frying Peanuts Decreases Soluble Peanut (Arachis Hypogaea) Allergens Ara h 1 and Ara h 2 But Does Not Generate Hypoallergenic Peanuts
- PMID: 27310538
- PMCID: PMC4911009
- DOI: 10.1371/journal.pone.0157849
Boiling and Frying Peanuts Decreases Soluble Peanut (Arachis Hypogaea) Allergens Ara h 1 and Ara h 2 But Does Not Generate Hypoallergenic Peanuts
Abstract
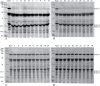
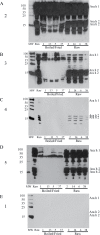
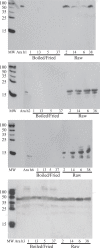
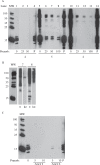
Peanut allergy continues to be a problem in most developed countries of the world. We sought a processing method that would alter allergenic peanut proteins, such that allergen recognition by IgE from allergic individuals would be significantly reduced or eliminated. Such a method would render accidental exposures to trace amounts of peanuts safer. A combination of boiling and frying decreased recovery of Ara h 1 and Ara h 2 at their expected MWs. In contrast, treatment with high pressures under varying temperatures had no effect on protein extraction profiles. Antibodies specific for Ara h 1, Ara h 2, and Ara h 6 bound proteins extracted from raw samples but not in boiled/fried samples. However, pre-incubation of serum with boiled/fried extract removed most raw peanut-reactive IgE from solution, including IgE directed to Ara h 1 and 2. Thus, this method of processing is unlikely to generate a peanut product tolerated by peanut allergic patients. Importantly, variability in individual patients' IgE repertoires may mean that some patients' IgE would bind fewer polypeptides in the sequentially processed seed.
Conflict of interest statement
Figures


References
-
- Burks AW, Williams LW, Helm RM, Connaughton C, Cockrell G, O'Brien T (1991) Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. J Allergy Clin Immunol 88: 172–179. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

