The diagnostic performance of serum MUC5AC for cholangiocarcinoma: A systematic review and meta-analysis
- PMID: 27310944
- PMCID: PMC4998430
- DOI: 10.1097/MD.0000000000003513
The diagnostic performance of serum MUC5AC for cholangiocarcinoma: A systematic review and meta-analysis
Erratum in
-
Erratum: Medicine, Volume 95, Issue 24: Erratum.Medicine (Baltimore). 2016 Aug 7;95(31):e5074. doi: 10.1097/01.md.0000490009.39850.74. eCollection 2016 Aug. Medicine (Baltimore). 2016. PMID: 31265618 Free PMC article.
-
Erratum: The diagnostic performance of serum MUC5AC for cholangiocarcinoma: A systematic review and meta-analysis: Erratum.Medicine (Baltimore). 2017 Feb 24;96(8):e6218. doi: 10.1097/MD.0000000000006218. eCollection 2017 Feb. Medicine (Baltimore). 2017. PMID: 31305751 Free PMC article.
Abstract
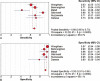
Specific diagnostic biomarker for cholangiocarcinoma (CCA) has been lacking. This systematic review and meta-analysis was performed aiming to investigate serum MUC5AC's diagnostic performance on CCA.Studies investigating serum MUC5AC's diagnostic value on CCA were retrieved from Pubmed, Embase, and Cochrane Library. The methodology quality of included studies was assessed according to QUADAS-2. Diagnostic 2 × 2 table was extracted from each eligible study, Meta-disc 1.4 was used for statistical analysis, data synthesis was done using a random-effects model. Subgroup analyses were conducted according to region and array method.Six eligible studies were identified, a total of 1213 patients were involved in the meta-analysis. The AUC on SROC was 0.9138, and the Q* was 8463. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio (DOR) were 0.69 (95% CI: 0.65-0.73), 0.93 (95% CI: 0.91-0.95), 8.99 (95% CI: 5.65-14.30), 0.33 (95% CI: 0.24-0.46), and 33.98 (95% CI: 20.12-57.40), respectively. Targeting MUC5AC's epitope has a higher pooled sensitivity than targeting MUC5AC protein (0.77 vs 0.63). There was substantial cross-study heterogeneity.Serum MUC5AC might be potentially used as a surrogate marker in the diagnosis of CCA. However, the appropriate array method and the optimum cut-off value are yet to be decided.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



References
-
- Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther 2006;23:1287–96. - PubMed
-
- Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis 2004;24:139–54. - PubMed
-
- Sasaki M, Tsuneyama K, Nakanuma Y. Aberrant expression of trefoil factor family 1 in biliary epithelium in hepatolithiasis and cholangiocarcinoma. Lab Invest 2003;83:1403–13. - PubMed
-
- Park SY, Roh SJ, Kim YN, et al. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact. Oncol Rep 2009;22:649–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

