Infliximab therapy for intestinal, neurological, and vascular involvement in Behcet disease: Efficacy, safety, and pharmacokinetics in a multicenter, prospective, open-label, single-arm phase 3 study
- PMID: 27310969
- PMCID: PMC4998455
- DOI: 10.1097/MD.0000000000003863
Infliximab therapy for intestinal, neurological, and vascular involvement in Behcet disease: Efficacy, safety, and pharmacokinetics in a multicenter, prospective, open-label, single-arm phase 3 study
Erratum in
-
Erratum: Medicine, Volume 95, Issue 24: Erratum.Medicine (Baltimore). 2016 Aug 7;95(31):e5074. doi: 10.1097/01.md.0000490009.39850.74. eCollection 2016 Aug. Medicine (Baltimore). 2016. PMID: 31265618 Free PMC article.
Abstract
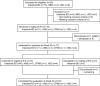
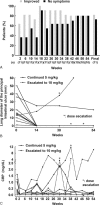
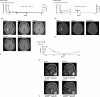
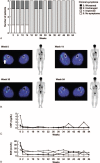
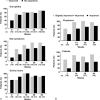
Behçet disease (BD) is a multisystem disease associated with a poor prognosis in cases of gastrointestinal, neurological, or vascular involvement. We conducted a multicenter, prospective, open-label, single-arm phase 3 study to determine the efficacy, safety, and pharmacokinetics of infliximab (IFX) in BD patients with these serious complications who had displayed poor response or intolerance to conventional therapy.IFX at 5 mg/kg was administered to 18 patients (11 intestinal BD, 3 neurological BD [NBD], and 4 vascular BD [VBD]) at weeks 0, 2, and 6 and every 8 weeks thereafter until week 46. In patients who showed inadequate responses to IFX after week 30, the dose was increased to 10 mg/kg. We then calculated the percentage of complete responders according to the predefined criteria depending on the symptoms and results of examinations (ileocolonoscopy, brain magnetic resonance imaging, computed tomography angiography, positron emission tomography, cerebrospinal fluid, or serum inflammatory markers), exploring the percentage of complete responders at week 30 (primary endpoint).The percentage of complete responders was 61% (11/18) at both weeks 14 and 30 and remained the same until week 54. Intestinal BD patients showed improvement in clinical symptoms along with decrease in C-reactive protein (CRP) levels after week 2. Consistently, scarring or healing of the principal ulcers was found in more than 80% of these patients after week 14. NBD patients showed improvement in clinical symptoms, imaging findings, and cerebrospinal fluid examinations. VBD patients showed improvement in clinical symptoms after week 2 with reductions in CRP levels and erythrocyte sedimentation rate. Imaging findings showed reversal of inflammatory changes in 3 of the 4 VBD patients. Irrespective of the type of BD, all patients achieved improvement in quality of life, leading to the dose reduction or withdrawal of steroids. IFX dose was increased to 10 mg/kg in 3 intestinal BD patients, resulting in the improvement of clinical symptoms, CRP levels, and visual analogue scale score. Safety and pharmacokinetics profiles were comparable to those in patients with rheumatoid arthritis or Crohn disease. These findings support IFX as a new therapeutic option for patients with intestinal BD, NBD, or VBD.
Figures


References
-
- Suzuki Kurokawa M, Suzuki N. Behcet's disease. Clin Exp Med 2004;3:10–20. - PubMed
-
- Ideguchi H, Suda A, Takeno M, et al. Behçet disease: evolution of clinical manifestations. Medicine 2011;90:125–32. - PubMed
-
- Pineton de Chambrun M, Wechsler B, Geri G, et al. New insights into the pathogenesis of Behçet's disease. Autoimmun Rev 2012;11:687–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

