Oral atenolol therapy for proliferating infantile hemangioma: A prospective study
- PMID: 27310994
- PMCID: PMC4998480
- DOI: 10.1097/MD.0000000000003908
Oral atenolol therapy for proliferating infantile hemangioma: A prospective study
Erratum in
-
Erratum: Medicine, Volume 95, Issue 24: Erratum.Medicine (Baltimore). 2016 Aug 7;95(31):e5074. doi: 10.1097/01.md.0000490009.39850.74. eCollection 2016 Aug. Medicine (Baltimore). 2016. PMID: 31265618 Free PMC article.
Abstract
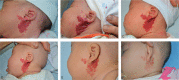
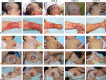
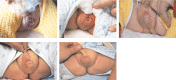
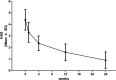
Propranolol, a lipophilic nonselective β-blocker, has recently been reported to be the treatment of choice for select types of infantile hemangiomas (IHs). Atenolol is a hydrophilic, selective β1-blocker and therefore may be not associated with side effects attributable to β2-adrenergic receptor blockade and lipophilicity. However, the efficacy and safety of atenolol in the treatment of IH are poorly understood. The aim of this study was to evaluate the efficacy and safety of atenolol in the treatment of proliferating IHs.A study of 76 infants between the ages of 5 to 20 weeks with superficial or mixed IH was conducted between August 2013 and March 2015. Oral atenolol was administered in a progressive schedule to 1 mg/kg per day in a single dose. Efficacy was assessed using the Hemangioma Activity Score (HAS) at weeks 0, 1, 4, 12, and 24. Safety was evaluated at weeks 0, 1, 4, 8, 12, 16, 20, and 24.In total, 70 patients completed 24 weeks of treatment. IH growth abruptly stopped for 93.4% of patients within the fourth week of treatment with atenolol. In ulcerated IHs, complete healing of the ulcerations occurred in an average treatment time of 5.5 weeks. Atenolol treatment promoted dramatic decreases in HAS scores after week 1. An "excellent" treatment response (compete or nearly complete resolution of the IH) was observed in 56.5% of patients at week 24. No significant hypoglycemia, bronchospasm, bradycardia, or hypotension occurred. The most common adverse event was diarrhea, followed by agitation and sleep disturbance.This study demonstrated that atenolol was effective and safe at a dose of 1 mg/kg per day for 24 weeks in the treatment of proliferating IHs.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
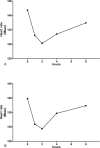
References
-
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics 2013;131:99–108. - PubMed
-
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics 2015;136:e1060–104. - PubMed
-
- George ME, Sharma V, Jacobson J, et al. Adverse effects of systemic glucocorticosteroid therapy in infants with hemangiomas. Arch Dermatol 2004;140:963–9. - PubMed
-
- Leaute-Labreze C, Dumas DLRE, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649–51. - PubMed
-
- Sans V, de la Roque ED, Berge J, et al. Propranolol for severe infantile hemangiomas: follow-up report. Pediatrics 2009;124:e423–31. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

