Adverse Outcome Pathways as Tools to Assess Drug-Induced Toxicity
- PMID: 27311472
- PMCID: PMC5436615
- DOI: 10.1007/978-1-4939-3609-0_14
Adverse Outcome Pathways as Tools to Assess Drug-Induced Toxicity
Abstract
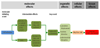
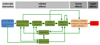
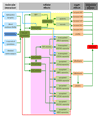
Adverse outcome pathways (AOPs) are novel tools in toxicology and human risk assessment with broad potential. AOPs are designed to provide a clear-cut mechanistic representation of toxicological effects that span over different layers of biological organization. AOPs share a common structure consisting of a molecular initiating event, a series of key events connected by key event relationships, and an adverse outcome. Development and evaluation of AOPs ideally complies with guidelines issued by the Organization for Economic Cooperation and Development. AOP frameworks have yet been proposed for major types of drug-induced injury, especially in the liver, including steatosis, fibrosis, and cholestasis. These newly postulated AOPs can serve a number of purposes pertinent to safety assessment of drugs, in particular the establishment of quantitative structure-activity relationships, the development of novel in vitro toxicity screening tests, and the elaboration of prioritization strategies.
Keywords: AOP; Cholestasis; Drug safety; Fibrosis; Steatosis.
Figures
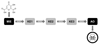
References
-
- OECD. Proposal for a template and guidance on developing and assessing the completeness of adverse outcome pathways. Series on Testing and Assessment. 2013;184:1–45.
-
- US EPA. Guidelines for carcinogen risk assessment. Washington D.C: 2005.
-
- Bogdanffy MS, Daston G, Faustman EM, et al. Harmonization of cancer and noncancer risk assessment: proceedings of a consensus-building workshop. Toxicol Sci. 2001;61:18–31. - PubMed
-
- Meek ME, Bucher JR, Cohen SM, et al. A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol. 2003;33:591–653. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

