Characteristics of the Clostridium difficile cell envelope and its importance in therapeutics
- PMID: 27311697
- PMCID: PMC5270738
- DOI: 10.1111/1751-7915.12372
Characteristics of the Clostridium difficile cell envelope and its importance in therapeutics
Abstract
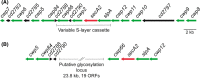
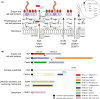
Clostridium difficile infection (CDI) is a challenging threat to human health. Infections occur after disruption of the normal microbiota, most commonly through the use of antibiotics. Current treatment for CDI largely relies on the broad-spectrum antibiotics vancomycin and metronidazole that further disrupt the microbiota resulting in frequent recurrence, highlighting the need for C. difficile-specific antimicrobials. The cell surface of C. difficile represents a promising target for the development of new drugs. C. difficile possesses a highly deacetylated peptidoglycan cell wall containing unique secondary cell wall polymers. Bound to the cell wall is an essential S-layer, formed of SlpA and decorated with an additional 28 related proteins. In addition to the S-layer, many other cell surface proteins have been identified, including several with roles in host colonization. This review aims to summarize our current understanding of these different C. difficile cell surface components and their viability as therapeutic targets.
© 2016 The Authors. Microbial Biotechnology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures

References
-
- Adamo, R. , Romano, M.R. , Berti, F. , Leuzzi, R. , Tontini, M. , Danieli, E. , et al (2012) Phosphorylation of the synthetic hexasaccharide repeating unit is essential for the induction of antibodies to Clostridium difficile PSII cell wall polysaccharide. ACS Chem Biol 7: 1420–1428. - PubMed
-
- Ammam, F. , Marvaud, J.C. , and Lambert, T. (2012) Distribution of the vanG‐like gene cluster in Clostridium difficile clinical isolates. Can J Microbiol 58: 547–551. - PubMed
-
- Ammam, F. , Meziane‐Cherif, D. , Mengin‐Lecreulx, D. , Blanot, D. , Patin, D. , Boneca, I.G. , et al (2013) The functional vanGCd cluster of Clostridium difficile does not confer vancomycin resistance. Mol Microbiol 89: 612–625. - PubMed
-
- Barketi‐Klai, A. , Hoys, S. , Lambert‐Bordes, S. , Collignon, A. , and Kansau, I. (2011) Role of fibronectin‐binding protein A in Clostridium difficile intestinal colonization. J Med Microbiol 60: 1155–1161. - PubMed
-
- Berti, F. , and Adamo, R. (2013) Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem Biol 8: 1653–1663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

