Type I interferon promotes alveolar epithelial type II cell survival during pulmonary Streptococcus pneumoniae infection and sterile lung injury in mice
- PMID: 27312374
- PMCID: PMC5370074
- DOI: 10.1002/eji.201546201
Type I interferon promotes alveolar epithelial type II cell survival during pulmonary Streptococcus pneumoniae infection and sterile lung injury in mice
Abstract
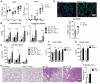
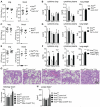
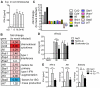
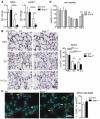
Protecting the integrity of the lung epithelial barrier is essential to ensure respiration and proper oxygenation in patients suffering from various types of lung inflammation. Type I interferon (IFN-I) has been associated with pulmonary epithelial barrier function, however, the mechanisms and involved cell types remain unknown. We aimed to investigate the importance of IFN-I with respect to its epithelial barrier strengthening function to better understand immune-modulating effects in the lung with potential medical implications. Using a mouse model of pneumococcal pneumonia, we revealed that IFN-I selectively protects alveolar epithelial type II cells (AECII) from inflammation-induced cell death. Mechanistically, signaling via the IFN-I receptor on AECII is sufficient to promote AECII survival. The net effects of IFN-I are barrier protection, together with diminished tissue damage, inflammation, and bacterial loads. Importantly, we found that the protective role of IFN-I can also apply to sterile acute lung injury, in which loss of IFN-I signaling leads to a significant reduction in barrier function caused by AECII cell death. Our data suggest that IFN-I is an important mediator in lung inflammation that plays a protective role by antagonizing inflammation-associated cell obstruction, thereby strengthening the integrity of the epithelial barrier.
Keywords: Acute lung injury; Alveolar epithelial cells; Epithelial barrier; Pneumococcal pneumonia; Type I interferon.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Qiu H, Fan Y, Joyee AG, Wang S, Han X, Bai H, Jiao L, et al. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J. Immunol. 2008;181:2092–2102. - PubMed
-
- Rothfuchs AG, Trumstedt C, Mattei F, Schiavoni G, Hidmark A, Wigzell H, Rottenberg ME. STAT1 regulates IFN-alpha beta- and IFN-gamma-dependent control of infection with Chlamydia pneumoniae by nonhemopoietic cells. J. Immunol. 2006;176:6982–6990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

