Early synaptic deficits in the APP/PS1 mouse model of Alzheimer's disease involve neuronal adenosine A2A receptors
- PMID: 27312972
- PMCID: PMC4915032
- DOI: 10.1038/ncomms11915
Early synaptic deficits in the APP/PS1 mouse model of Alzheimer's disease involve neuronal adenosine A2A receptors
Abstract
Synaptic plasticity in the autoassociative network of recurrent connections among hippocampal CA3 pyramidal cells is thought to enable the storage of episodic memory. Impaired episodic memory is an early manifestation of cognitive deficits in Alzheimer's disease (AD). In the APP/PS1 mouse model of AD amyloidosis, we show that associative long-term synaptic potentiation (LTP) is abolished in CA3 pyramidal cells at an early stage. This is caused by activation of upregulated neuronal adenosine A2A receptors (A2AR) rather than by dysregulation of NMDAR signalling or altered dendritic spine morphology. Neutralization of A2AR by acute pharmacological inhibition, or downregulation driven by shRNA interference in a single postsynaptic neuron restore associative CA3 LTP. Accordingly, treatment with A2AR antagonists reverts one-trial memory deficits. These results provide mechanistic support to encourage testing the therapeutic efficacy of A2AR antagonists in early AD patients.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


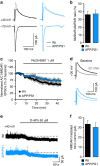


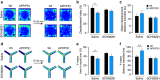

References
-
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann. N. Y. Acad. Sci. 1097, 146–155 (2007). - PubMed
-
- Selkoe D. J. Alzheimer's disease is a synaptic failure. Science 298, 789–791 (2002). - PubMed
-
- Nakazawa K. et al. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38, 305–315 (2003). - PubMed
-
- Rolls E. T. & Treves A. The neuronal encoding of information in the brain. Prog. Neurobiol. 95, 448–490 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

