T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4⁺ T cells
- PMID: 27313051
- PMCID: PMC5015487
- DOI: 10.1126/science.aad1210
T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4⁺ T cells
Abstract
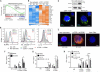
The NLRP3 inflammasome controls interleukin-1β maturation in antigen-presenting cells, but a direct role for NLRP3 in human adaptive immune cells has not been described. We found that the NLRP3 inflammasome assembles in human CD4(+) T cells and initiates caspase-1-dependent interleukin-1β secretion, thereby promoting interferon-γ production and T helper 1 (T(H)1) differentiation in an autocrine fashion. NLRP3 assembly requires intracellular C5 activation and stimulation of C5a receptor 1 (C5aR1), which is negatively regulated by surface-expressed C5aR2. Aberrant NLRP3 activity in T cells affects inflammatory responses in human autoinflammatory disease and in mouse models of inflammation and infection. Our results demonstrate that NLRP3 inflammasome activity is not confined to "innate immune cells" but is an integral component of normal adaptive T(H)1 responses.
Copyright © 2016, American Association for the Advancement of Science.
Figures





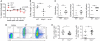
Comment in
-
Blurring the Lines Between Innate and Adaptive Immunity.Transplantation. 2016 Sep;100(9):1789-1790. doi: 10.1097/TP.0000000000001429. Transplantation. 2016. PMID: 27542068 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

