Tissue-specific down-regulation of S-adenosyl-homocysteine via suppression of dAhcyL1/dAhcyL2 extends health span and life span in Drosophila
- PMID: 27313316
- PMCID: PMC4926864
- DOI: 10.1101/gad.282277.116
Tissue-specific down-regulation of S-adenosyl-homocysteine via suppression of dAhcyL1/dAhcyL2 extends health span and life span in Drosophila
Abstract
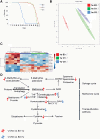
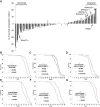
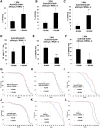
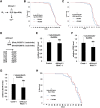
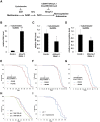
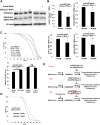
Aging is a risk factor for many human pathologies and is characterized by extensive metabolic changes. Using targeted high-throughput metabolite profiling in Drosophila melanogaster at different ages, we demonstrate that methionine metabolism changes strikingly during aging. Methionine generates the methyl donor S-adenosyl-methionine (SAM), which is converted via methylation to S-adenosyl-homocysteine (SAH), which accumulates during aging. A targeted RNAi screen against methionine pathway components revealed significant life span extension in response to down-regulation of two noncanonical Drosophila homologs of the SAH hydrolase Ahcy (S-adenosyl-L-homocysteine hydrolase [SAHH[), CG9977/dAhcyL1 and Ahcy89E/CG8956/dAhcyL2, which act as dominant-negative regulators of canonical AHCY. Importantly, tissue-specific down-regulation of dAhcyL1/L2 in the brain and intestine extends health and life span. Furthermore, metabolomic analysis of dAhcyL1-deficient flies revealed its effect on age-dependent metabolic reprogramming and H3K4 methylation. Altogether, reprogramming of methionine metabolism in young flies and suppression of age-dependent SAH accumulation lead to increased life span. These studies highlight the role of noncanonical Ahcy enzymes as determinants of healthy aging and longevity.
Keywords: CG8956/Ahcy89E/AhcyL2; CG9977/AhcyL1; S-adenosyl-homocysteine (SAH); aging; life span; methionine restriction.
© 2016 Parkhitko et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

References
-
- Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. 2006. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell 22: 795–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
