Mechanotransduction activates canonical Wnt/β-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves
- PMID: 27313318
- PMCID: PMC4926867
- DOI: 10.1101/gad.282400.116
Mechanotransduction activates canonical Wnt/β-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves
Abstract
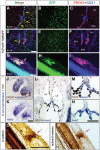
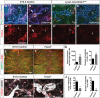
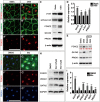
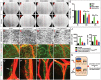
Lymphatic vasculature regulates fluid homeostasis by returning interstitial fluid to blood circulation. Lymphatic endothelial cells (LECs) are the building blocks of the entire lymphatic vasculature. LECs originate as a homogeneous population of cells predominantly from the embryonic veins and undergo stepwise morphogenesis to become the lymphatic capillaries, collecting vessels or valves. The molecular mechanisms underlying the morphogenesis of the lymphatic vasculature remain to be fully understood. Here we show that canonical Wnt/β-catenin signaling is necessary for lymphatic vascular morphogenesis. Lymphatic vascular-specific ablation of β-catenin in mice prevents the formation of lymphatic and lymphovenous valves. Additionally, lymphatic vessel patterning is defective in these mice, with abnormal recruitment of mural cells. We found that oscillatory shear stress (OSS), which promotes lymphatic vessel maturation, triggers Wnt/β-catenin signaling in LECs. In turn, Wnt/β-catenin signaling controls the expression of several molecules, including the lymphedema-associated transcription factor FOXC2. Importantly, FOXC2 completely rescues the lymphatic vessel patterning defects in mice lacking β-catenin. Thus, our work reveals that mechanical stimulation is a critical regulator of lymphatic vascular development via activation of Wnt/β-catenin signaling and, in turn, FOXC2.
Keywords: FOXC2; PROX1; Wnt/β-catenin signaling; lymphatic valves; lymphatic vascular development; lymphovenous valves.
© 2016 Cha et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Berger J, Berger S, Tuoc TC, D'Amelio M, Cecconi F, Gorski JA, Jones KR, Gruss P, Stoykova A. 2007. Conditional activation of Pax6 in the developing cortex of transgenic mice causes progenitor apoptosis. Development 134: 1311–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
