A Novel Malaria Pf/Pv Ab Rapid Diagnostic Test Using a Differential Diagnostic Marker Identified by Network Biology
- PMID: 27313496
- PMCID: PMC4910601
- DOI: 10.7150/ijbs.14408
A Novel Malaria Pf/Pv Ab Rapid Diagnostic Test Using a Differential Diagnostic Marker Identified by Network Biology
Abstract
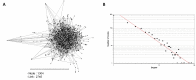
Rapid diagnostic tests (RDTs) can detect anti-malaria antibodies in human blood. As they can detect parasite infection at the low parasite density, they are useful in endemic areas where light infection and/or re-infection of parasites are common. Thus, malaria antibody tests can be used for screening bloods in blood banks to prevent transfusion-transmitted malaria (TTM), an emerging problem in malaria endemic areas. However, only a few malaria antibody tests are available in the microwell-based assay format and these are not suitable for field application. A novel malaria antibody (Ab)-based RDT using a differential diagnostic marker for falciparum and vivax malaria was developed as a suitable high-throughput assay that is sensitive and practical for blood screening. The marker, merozoite surface protein 1 (MSP1) was discovered by generation of a Plasmodium-specific network and the hierarchical organization of modularity in the network. Clinical evaluation revealed that the novel Malaria Pf/Pv Ab RDT shows improved sensitivity (98%) and specificity (99.7%) compared with the performance of a commercial kit, SD BioLine Malaria P.f/P.v (95.1% sensitivity and 99.1% specificity). The novel Malaria Pf/Pv Ab RDT has potential for use as a cost-effective blood-screening tool for malaria and in turn, reduces TTM risk in endemic areas.
Keywords: Blood screening; MSP1; Malaria antibody test; Transfusion-transmitted malaria.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


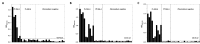

References
-
- WHO. World malaria report: 2013. 2013.
-
- WHO. Information note on recommended selection criteria for procurement of malaria rapid diagnostic tests. 2014.
-
- Mouatcho JC, Goldring JP. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62:1491–505. - PubMed
-
- Proux S, Hkirijareon L, Ngamngonkiri C, McConnell S, Nosten F. Paracheck-Pf: a new, inexpensive and reliable rapid test for P. falciparum malaria. Trop Med Int Health. 2001;6:99–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

