A Pilot Study of 18F-FLT PET/CT in Pediatric Lymphoma
- PMID: 27313888
- PMCID: PMC4899586
- DOI: 10.1155/2016/6045894
A Pilot Study of 18F-FLT PET/CT in Pediatric Lymphoma
Abstract
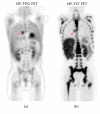
We performed an observational pilot study of 18F-FLT PET/CT in pediatric lymphoma. Eight patients with equivocal 18F-FDG PET/CT underwent imaging with 18F-FLT PET/CT. No immediate adverse reactions to 18F-FLT were observed. Compared to 18F-FDG, 18F-FLT uptake was significantly higher in bone marrow and liver (18F-FLT SUV 8.6 ± 0.6 and 5.0 ± 0.3, versus 18F-FDG SUV 1.9 ± 0.1 and 3.4 ± 0.7, resp., p < 0.05). In total, 15 lesions were evaluated with average 18F-FDG and 18F-FLT SUVs of 2.6 ± 0.1 and 2.0 ± 0.4, respectively. Nonspecific uptake in reactive lymph nodes and thymus was observed. Future studies to assess the clinical utility of 18F-FLT PET/CT in pediatric lymphoma are planned.
Figures


References
-
- Gilles R., Vogel W. V., Gidding C. E. M., Janssens G. O. R. J., Van Der Vliet T. M., Oyen W. J. G. 18F-fluoro-L-thymidine-PET for the evaluation of primary brain tumours in children: a report of three cases. Nuclear Medicine Communications. 2010;31(6):482–487. doi: 10.1097/mnm.0b013e328318dc18. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

