Protons and Hydroxide Ions in Aqueous Systems
- PMID: 27314430
- PMCID: PMC7116074
- DOI: 10.1021/acs.chemrev.5b00736
Protons and Hydroxide Ions in Aqueous Systems
Abstract
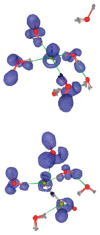
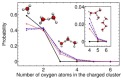
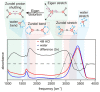
Understanding the structure and dynamics of water's constituent ions, proton and hydroxide, has been a subject of numerous experimental and theoretical studies over the last century. Besides their obvious importance in acid-base chemistry, these ions play an important role in numerous applications ranging from enzyme catalysis to environmental chemistry. Despite a long history of research, many fundamental issues regarding their properties continue to be an active area of research. Here, we provide a review of the experimental and theoretical advances made in the last several decades in understanding the structure, dynamics, and transport of the proton and hydroxide ions in different aqueous environments, ranging from water clusters to the bulk liquid and its interfaces with hydrophobic surfaces. The propensity of these ions to accumulate at hydrophobic surfaces has been a subject of intense debate, and we highlight the open issues and challenges in this area. Biological applications reviewed include proton transport along the hydration layer of various membranes and through channel proteins, problems that are at the core of cellular bioenergetics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

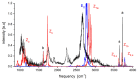
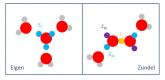
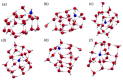

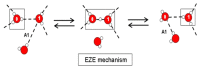

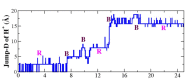
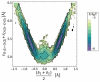
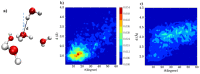
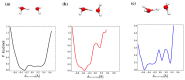






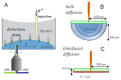
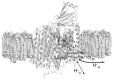

References
-
- Kreuer KD. Proton Conductivity: Materials and Applications. Chem Mater. 1996;8:610–641.
-
- Cukierman S. Et Tu, Grotthuss! And Other Unfinished Stories. Biochim Biophys Acta Bioenerg. 2006;1757:876–885. - PubMed
-
- Wraight CA. Chance and Design - Proton Transfer in Water, Channels and Bioenergetic Proteins. Biochim Biophys Acta Bioenerg. 2006;1757:886–912. - PubMed
-
- Marx D, Chandra A, Tuckerman ME. Aqueous Basic Solutions: Hydroxide Solvation, Structural Diffusion, and Comparison to the Hydrated Proton. Chem Rev. 2010;110:2174–2216. - PubMed
-
- Knight C, Voth GA. The Curious Case of the Hydrated Proton. Acc Chem Res. 2012;45:101–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

