Hydrogen Sulfide Is an Antiviral and Antiinflammatory Endogenous Gasotransmitter in the Airways. Role in Respiratory Syncytial Virus Infection
- PMID: 27314446
- PMCID: PMC5105180
- DOI: 10.1165/rcmb.2015-0385OC
Hydrogen Sulfide Is an Antiviral and Antiinflammatory Endogenous Gasotransmitter in the Airways. Role in Respiratory Syncytial Virus Infection
Abstract
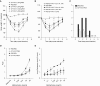
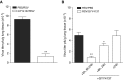
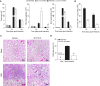
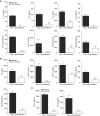
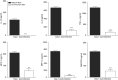
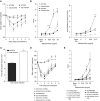
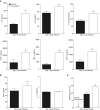
Hydrogen sulfide (H2S) is an endogenous gaseous transmitter whose role in the pathophysiology of several lung diseases has been increasingly appreciated. Our recent studies in vitro have shown, we believe for the first time, that H2S has an important antiviral and antiinflammatory activity in respiratory syncytial virus (RSV) infection, the leading cause of bronchiolitis and viral pneumonia in children. Our objective was to evaluate the therapeutic potential of GYY4137, a novel slow-releasing H2S donor, for the prevention and treatment of RSV-induced lung disease, as well as to investigate the role of endogenous H2S in a mouse model of RSV infection. Ten- to 12-week-old BALB/c mice treated with GYY4137, or C57BL/6J mice genetically deficient in the cystathionine γ-lyase enzyme, the major H2S-generating enzyme in the lung, were infected with RSV and assessed for viral replication, clinical disease, airway hyperresponsiveness, and inflammatory responses. Our results show that intranasal delivery of GYY4137 to RSV-infected mice significantly reduced viral replication and markedly improved clinical disease parameters and pulmonary dysfunction compared with the results in vehicle-treated control mice. The protective effect of the H2S donor was associated with a significant reduction of viral-induced proinflammatory mediators and lung cellular infiltrates. Furthermore, cystathionine γ-lyase-deficient mice showed significantly enhanced RSV-induced lung disease and viral replication compared with wild-type animals. Overall, our results indicate that H2S exerts a novel antiviral and antiinflammatory activity in the context of RSV infection and represent a potential novel pharmacological approach for ameliorating virus-induced lung disease.
Keywords: airway hyperresponsiveness; antiviral; cystathionine γ-lyase; lung injury; paramyxovirus.
Figures
Similar articles
-
Role of hydrogen sulfide in paramyxovirus infections.J Virol. 2015 May;89(10):5557-68. doi: 10.1128/JVI.00264-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740991 Free PMC article.
-
Thiol-Activated Hydrogen Sulfide Donors Antiviral and Anti-Inflammatory Activity in Respiratory Syncytial Virus Infection.Viruses. 2018 May 10;10(5):249. doi: 10.3390/v10050249. Viruses. 2018. PMID: 29747463 Free PMC article.
-
Cystathionine γ-lyase deficiency enhances airway reactivity and viral-induced disease in mice exposed to side-stream tobacco smoke.Pediatr Res. 2019 Jul;86(1):39-46. doi: 10.1038/s41390-019-0396-6. Epub 2019 Apr 15. Pediatr Res. 2019. PMID: 30986815 Free PMC article.
-
Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis.Int J Cardiol. 2014 Mar 15;172(2):313-7. doi: 10.1016/j.ijcard.2014.01.068. Epub 2014 Jan 24. Int J Cardiol. 2014. PMID: 24491853 Review.
-
H2S Donors and Their Use in Medicinal Chemistry.Biomolecules. 2021 Dec 18;11(12):1899. doi: 10.3390/biom11121899. Biomolecules. 2021. PMID: 34944543 Free PMC article. Review.
Cited by
-
The role of host defences in Covid 19 and treatments thereof.Mol Med. 2020 Sep 29;26(1):90. doi: 10.1186/s10020-020-00216-9. Mol Med. 2020. PMID: 32993497 Free PMC article. Review.
-
Anti-inflammatory and antiviral roles of hydrogen sulfide: Rationale for considering H2 S donors in COVID-19 therapy.Br J Pharmacol. 2020 Nov;177(21):4931-4941. doi: 10.1111/bph.15230. Epub 2020 Aug 26. Br J Pharmacol. 2020. PMID: 32783196 Free PMC article. Review.
-
Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection.Viruses. 2019 Aug 8;11(8):732. doi: 10.3390/v11080732. Viruses. 2019. PMID: 31398832 Free PMC article.
-
Hydrogen Sulfide: A Novel Player in Airway Development, Pathophysiology of Respiratory Diseases, and Antiviral Defenses.Am J Respir Cell Mol Biol. 2017 Oct;57(4):403-410. doi: 10.1165/rcmb.2017-0114TR. Am J Respir Cell Mol Biol. 2017. PMID: 28481637 Free PMC article. Review.
-
Hydrogen sulfide regulates bone remodeling and promotes orthodontic tooth movement.Mol Med Rep. 2017 Dec;16(6):9415-9422. doi: 10.3892/mmr.2017.7813. Epub 2017 Oct 17. Mol Med Rep. 2017. PMID: 29039565 Free PMC article.
References
-
- Chen Y, Wang R. The message in the air: hydrogen sulfide metabolism in chronic respiratory diseases. Respir Physiol Neurobiol. 2012;184:130–138. - PubMed
-
- Wang P, Zhang G, Wondimu T, Ross B, Wang R. Hydrogen sulfide and asthma. Exp Physiol. 2011;96:847–852. - PubMed
-
- Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. - PubMed
-
- Wang R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens. 2011;20:107–112. - PubMed
-
- Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

