Topical cystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease
- PMID: 27314455
- PMCID: PMC8682957
- DOI: 10.1002/14651858.CD005599.pub5
Topical cystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease
Abstract
Background: Cystic fibrosis is caused by a defective gene encoding a protein called the cystic fibrosis transmembrane conductance regulator (CFTR), and is characterised by chronic lung infection resulting in inflammation and progressive lung damage that results in a reduced life expectancy.
Objectives: To determine whether topical CFTR gene replacement therapy to the lungs in people with cystic fibrosis is associated with improvements in clinical outcomes, and to assess any adverse effects.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches, handsearching relevant journals and abstract books of conference proceedings.Date of most recent search: 05 May 2016.An additional search of the National Institutes for Health (NIH) Genetic Modification Clinical Research Information System (GeMCRIS) was also performed for the years 1992 to 2015.Date of most recent search: 20 April 2016.
Selection criteria: Randomised controlled studies comparing topical CFTR gene delivery to the lung, using either viral or non-viral delivery systems, with placebo or an alternative delivery system in people with confirmed cystic fibrosis.
Data collection and analysis: The authors independently extracted data and assessed study quality. Authors of included studies were contacted and asked for any available additional data. Meta-analysis was limited due to differing study designs.
Main results: Four randomised controlled studies met the inclusion criteria for this review, involving a total of 302 participants lasting from 29 days to 13 months; 14 studies were excluded. The included studies differed in terms of CFTR gene replacement agent and study design, which limited the meta-analysis. One study only enrolled adult males, the remaining studies included both males and females aged 12 years and over.Risk of bias in the studies was moderate. Random sequence generation and allocation concealment was only described in the more recent study; the remaining three studies were judged to have an unclear risk of bias. All four studies documented double-blinding to the intervention, but there is some uncertainty with regards to participant blinding in one study. Some outcome data were missing from all four studies.There were no differences in either the number of respiratory exacerbations or the number of participants with an exacerbation between replacement therapy or placebo groups at any time point. Meta-analysis of most respiratory function tests showed no difference between treatment and placebo groups, but the smallest study (n = 16) reported forced vital capacity (litres) increased more in the placebo group at up to 24 hours. A further study reported a significant improvement in forced expiratory volume at one second (litres) at 30 days after participants had received their first dose of favouring the gene therapy agent, but this finding was not confirmed when combined with at second study in the meta-analysis. The more recent study (n = 140) demonstrated a small improvement in forced vital capacity (per cent predicted) at two and three months and again at 11 and 12 months for participants receiving CFTR gene replacement therapy compared to those receiving placebo. The same study reported a significant difference in the relative change in forced expiratory volume at one second (per cent predicted) at two months, three months and 12 months.One small study reported significant concerns with "influenza-like" symptoms in participants treated with CFTR gene replacement therapy; this was not reported on repeated use of the same agent in a larger recent study.There was no other evidence of positive impact on outcomes, in particular improved quality of life or reduced treatment burden.Two studies measured ion transport in the lower airways; one (n = 16) demonstrated significant changes toward normal values in the participants who received gene transfer agents (P < 0.0001), mean difference 6.86 (95% confidence interval 3.77 to 9.95). The second study (n = 140) also reported significant changes toward normal values (P = 0.032); however, aggregate data were not available for analysis. In the most recent study, there was also evidence of increased salt transport in cells obtained by brushing the lower airway. These outcomes, whilst important, are not of direct clinical relevance.
Authors' conclusions: One study of liposome-based CFTR gene transfer therapy demonstrated some improvements in respiratory function in people with CF, but this limited evidence of efficacy does not support this treatment as a routine therapy at present. There was no evidence of efficacy for viral-mediated gene delivery.Future studies need to investigate clinically important outcome measures.
Conflict of interest statement
All authors: none known.
Figures











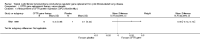

Update of
-
Topical cystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease.Cochrane Database Syst Rev. 2013 Nov 26;(11):CD005599. doi: 10.1002/14651858.CD005599.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Jun 17;(6):CD005599. doi: 10.1002/14651858.CD005599.pub5. PMID: 24282073 Updated.
References
References to studies included in this review
Alton 1999 {published data only}
-
- Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, et al. Cationic lipid‐mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double‐blind placebo‐controlled trial. The Lancet 1999;353(9157):947‐54. [CFGD Register: BD11a] - PubMed
-
- Davies MG, Stern M, Geddes DM, Alton EWFW. Reduction in sputum inflammatory cells and IL‐8 following pulmonary administration of liposome mediated CFTR gene transfer in CF subjects [abstract]. Thorax 1998;53(Suppl 4):A14. [CFGD Register: BD11b]
-
- Stern M, Phillips J, Jaffe A, Farley R, Chadwick S, Davies J. A double blind placebo controlled trial of pulmonary and nasal administration of liposome‐mediated CFTR gene transfer in CF subjects [abstract]. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A564. [CFGD Register: BD11d]
-
- Stern M, Phillips J, Jaffe A, Farley R, Chadwick S, Davies J, et al. A double blind placebo controlled trial of pulmonary and nasal administration of liposome‐mediated CFTR gene transfer in CF subjects [abstract]. Thorax 1997;52(Suppl 6):A3. [CFGD Register: BD11c]
Alton 2015 {published data only}
-
- Clinical study protocol ‐ a randomised, double‐blind, placebo‐controlled phase 2b clinical trial of repeated application of gene therapy in patients with cystic fibrosis. Short title: repeated application of gene therapy in CF patients. Version: 3. Date 26.03.2012 [online]. http://www.eme.ac.uk/funded_projects/PDFs/111425protocol.pdf 2013. [CFGD Register: BD180d; ]
-
- Project brief: A randomised double‐blind placebo controlled phase 2b clinical trial of repeated application of gene therapy in patients with cystic fibrosis [online]. http://www.eme.ac.uk/funded_projects/PDFs/111425info.pdf 2013. [CFGD Register: BD180c; ] - PubMed
-
- Alton E. The UK Gene Therapy Trial [abstract]. Pediatric Pulmonology 2014;49 Suppl 38:153, Abstract no: S10.1. [CFGD Register: BD180i]
-
- Alton E, Armstrong D, Bayfield K, Bilton D, Boyd A, Cheng S, et al. A randomized, double‐blind, placebo‐controlled trial of repeated nebulisations of non‐viral CFTR gene therapy in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 2015;50 Suppl 41:262, Abstract no: 192. [CENTRAL: 1092195; CFGD Register: BD180m; CRS: 5500135000001384]
-
- Alton EW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, et al. Repeated nebulisation of non‐viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double‐blind, placebo‐controlled, phase 2b trial. The Lancet. Respiratory Medicine 2015;3(9):684‐91. [CFGD Register: BD180j; CRS: 5500135000000005; PUBMED: 26149841] - PMC - PubMed
Moss 2004 {published data only}
-
- Moss RB, Aitken M, Clancy J, Milla C, Rodman D, Spencer T, et al. A multicenter, double‐blind, placebo controlled, phase II study of aerosolized TGAAVCF in cystic fibrosis patients with mild lung disease [abstract]. Pediatric Pulmonology 2002;34(Suppl 24):250‐1. [CFGD Register: BD17a]
-
- Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D, et al. Repeated adeno‐associated virus serotype 2 aerosol‐mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double‐blind, placebo‐controlled trial. Chest 2004;125(2):509‐21. [CFGD Register: BD17b] - PubMed
Moss 2007 {published and unpublished data}
-
- Moss RB, Heald AE, 25E01 SG. Phase 2B Trial of Aerosolized tgAAVCF for Cystic Fibrosis [abstract]. Journal of Cystic Fibrosis 2005;4 Suppl:S27. [CFGD Register: BD20b; ]
-
- Moss RB, Heald AE, 25E01 Study Group. A randomized phase 2B study of aerosolized tgAAVCF for treatment of cystic fibrosis [abstract]. American Thoracic Society International Conference; 2005 May 20‐25; San Diego, USA. 2005:A179. [CFGD Register: BD20c]
-
- Moss RB, Milla C, Colombo J, Accurso F, Zeitlin PL, Clancy JP, et al. Repeated aerosolized AAV‐CFTR for treatment of cystic fibrosis: a randomized placebo‐controlled phase 2B trial. Human Gene Therapy 2007;18(8):726‐32. [CFGD Register: BD20d] - PubMed
-
- Moss RB, Milla C, Colombo J, Clancy JP, Zeitlin P, Spencer T, et al. A multicenter, double‐blind, placebo‐controlled, phase 2b study of aerosolized TGAAVCF for the treatment of cystic fibrosis [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):257. [CFGD Register: BD20a]
References to studies excluded from this review
Davies 2011 {published data only}
-
- Alton E. Single dose of PGM169/GL67A in CF patients. http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=7094 Accessed on 22 September 2009. [UKCRN ID 7094]
-
- Alton E, Davies JC, Davies G, Lees B, Mohamedhossen MH, Soussi S, et al. Safety evaluation of a single escalating dose of pGM169/GL67A in the nose and lung of individuals with cystic fibrosis. www.rbht.nhs.uk/research/projects/?entryid31=161410&catid=651&p=1 Accessed 22 September 2009. [Royal Brompton & Harefield NHS Trust Ref No.: 2007CF011B]
-
- Davies JC, Davies G, Gill DR, Hyde SC, Boyd AC, Innes AJ, et al. Safety & expression of a single dose of lipid‐mediated CFTR gene therapy to the upper & lower airways of patients with CF. Pediatric Pulmonology 2011;46(Suppl 34):Abstract 198.
Flotte 1996 {published data only}
-
- Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, et al. A phase I study of an adeno‐associated virus‐CFTR gene vector in adult CF patients with mild lung disease. Human Gene Therapy 1996;7(9):1145‐59. [CFGD Register: BD6; MEDLINE: ] - PubMed
Gill 1997 {published data only}
-
- Gill DR, Southern KW, Mofford KA, Seddon T, Huang L, Sorgi F, et al. A placebo‐controlled study of liposome‐mediated gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Therapy 1997;4(3):199‐209. [CFGD Register: BD4c; MEDLINE: ] - PubMed
-
- Gill DR, Southern KW, Mofford KA, Sorgi F, Huang L, Higgins CF, et al. A Phase I clinical trial to assess the safety of DNA/liposome mediated gene transfer [abstract]. Tenth Annual North American Cystic Fibrosis Conference 1996;Suppl 13:264. [CFGD Register: BD4a; ]
-
- Hyde SC, Southern KW, Mofford KA, Sorgi F, Huang L, Higgins CF, et al. Towards DNA/liposome gene therapy for cystic fibrosis: A phase I clinical trial [abstract]. Pediatric Pulmonology 1996;22(Suppl 13):265. [CFGD Register: BD4b]
Harvey 1999 {published data only}
-
- Harvey BG, Leopold PL, Hackett NR, Grasso TM, Williams PM, Tucker AL, et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus see comments. Journal of Clinical Investigation 1999;104(9):1245‐55. [CFGD Register: BD13] - PMC - PubMed
Hyde 2000 {published data only}
-
- Hyde SC, Southern KW, Gileadi U, Fitzjohn EM, Mofford KA, Waddell BE, et al. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Therapy 2000;7(13):1156‐65. [CFGD Register: BD15b] - PubMed
-
- Southern KW, Hyde SC, Fitzjohn EM, Waddell BE, Egan JJ, Cole JY, et al. Repeated nasal administration of liposome‐mediated CFTR gene transfer reagents; the clinical and immunological consequences [abstract]. Pediatric Pulmonology 1997;24(Suppl 14):265. [CFGD Register: BD15a]
Joseph 2001 {published data only}
-
- Joseph PM, O'Sullivan BP, Lapey A, Dorkin H, Oren J, Balfour R, et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Human Gene Therapy 2001;12(11):1369‐82. [CFGD Register: BD16] - PubMed
Knowles 1995 {published data only}
-
- Knowles MR, Hohneker KW, Zhou Z, Olsen JC, Noah TL, Hu PC, et al. A controlled study of adenoviral‐vector‐mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. New England Journal of Medicine 1995;333(13):823‐31. [CFGD Register: BD1] - PubMed
Noone 2000 {published data only}
-
- Noone PG, Hohneker KW, Gipson CA, Schwartzbach CJ, Efthimiou J, Johnson LG, et al. Safety and biological efficacy of a lipid‐CFTR complex for gene transfer in the nasal epithelium of adult patients with cystic fibrosis [abstract]. Pediatric Pulmonology 1998;26(Suppl 17):260. [CFGD Register: BD10a] - PubMed
-
- Noone PG, Hohneker KW, Zhou Z, Johnson LG, Foy C, Gipson C, et al. Safety and biological efficacy of a lipid‐CFTR complex for gene transfer in the nasal epithelium of adult patients with cystic fibrosis. Molecular Therapy 2000;1(1):105‐14. [CFGD Register: BD10b] - PubMed
Porteous 1997 {published data only}
-
- Porteous DJ, Dorin JR, McLachlan G, Davidson Smith H, Davidson H, Stevenson BJ, et al. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Therapy 1997;4(3):210‐8. [CFGD Register: BD5b; MEDLINE: ] - PubMed
-
- Porteous DJ, Dorin JR, McLachlan G, Davidson‐Smith H, Davidson H, Stevenson BJ, et al. DOTAP catonic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis [abstract]. Pediatric Pulmonology 1996;22(Suppl 13):266. [CFGD Register: BD5a]
Wagner 1999 {published data only}
-
- Wagner JA, Messner AH, Moran ML, Daifuku R, Kouyama K, Desch JK, et al. Safety and biological efficacy of an adeno‐associated virus vector ‐ cystic fibrosis transmembrane regulator (AAV‐CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 1999;109(2 Pt 1):266‐74. [CFGD Register: BD8b] - PubMed
-
- Wagner JA, Nepomuceno IB, Shah N, Messner AH, Moran ML, Norbash AM, et al. Maxillary sinusitis as a surrogate model for CF gene therapy clinical trials in patients with antrostomies. Journal of Gene Medicine 1999;1(1):13‐21. [CFGD Register: BD8c] - PubMed
-
- Wagner JA, Reynolds T, Moran ML, Moss RB, Wine JJ, Flotte TR, et al. Efficient and persistent gene transfer of AAV‐CFTR in maxillary sinus [letter]. Lancet 1998;351(9117):1702‐3. [CFGD Register: BD8a] - PubMed
Wagner 2002 {published data only}
-
- Wagner JA, Messner AH, Friborg S, Reynolds T, Guggino WB, Moss RB, et al. A phase II, double‐blind, randomized, placebo‐controlled clinical trial of tgAAVCF in the maxillary sinus of CF patients [abstract]. Pediatric Pulmonology 1998;26(Suppl 17):260. [CFGD Register: BD9a]
-
- Wagner JA, Messner AH, Moran ML, Guggino WB, Flotte TR, Wine JJ, et al. A phase II, double‐blind, randomized, placebo‐controlled clinical trial of tgAAVCF using maxillary sinus delivery in CF patients with antrostomies [abstract]. Pediatric Pulmonology 1999;28(Suppl 19):223‐4. [CFGD Register: BD9b]
-
- Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, et al. A phase II, double‐blind, randomized, placebo‐controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Human Gene Therapy 2002;13(11):1349‐59. [CFGD Register: BD9c] - PubMed
Zabner 1996 {published data only}
-
- Zabner J, Ramsey BW, Meeker DP, Aitken ML, Balfour RP, Gibson RL, et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. Journal of Clinical Investigation 1996;97(6):1504‐11. [CFGD Register: BD18] - PMC - PubMed
Zabner 1997 {published data only}
Zuckerman 1999 {published data only}
-
- Zuckerman JB, Robinson CB, McCoy KS, Shell R, Sferra TJ, Chirmule N, et al. A phase I study of adenovirus‐mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Human Gene Therapy 1999;10(18):2973‐85. [CFGD Register: BD14] - PubMed
Additional references
Bobadilla 2002
-
- Bobadilla JL, Macek M, Fine JP, Farrell PM. Cystic Fibrosis: A worldwide analysis of CFTR mutations ‐ correlation with incidence data and application to screening. Human Mutation 2002;19(6):575‐606. - PubMed
Boucher 2004
-
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. European Respiratory Journal 2004;23(1):146‐58. - PubMed
Conway 1994
Deeks 2011
-
- Deeks J, Higgins J, Altman D on behalf of the Cochrane Statistical Methods Group (editors). Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. - PubMed
Ferrari 2002
FitzSimmons 1993
-
- FitzSimmons SC. The changing epidemiology of cystic fibrosis. Journal of Pediatrics 1993;122(1):1‐9. - PubMed
Frederiksen 1996
-
- Frederiksen B, Lanng S, Koch C, Hoiby N. Improved survival in the Danish centre treated cystic fibrosis patients: Results of aggressive treatment. Pediatric Pulmonology 1996;21(3):153‐8. - PubMed
Griesenbach 2004
Griesenbach 2005
-
- Griesenbach U, Boyd AC. Pre‐clinical and clinical endpoint assays for cystic fibrosis gene therapy. Journal of Cystic Fibrosis 2005;4:89‐100. - PubMed
Griesenbach 2008
-
- Griesenbach U. The UK gene therapy consortium research programme [abstract]. Journal of Cystic Fibrosis 2008;7(Suppl 3):S4.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jüni 2001
Lee 2005
Matsui 1998
-
- Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis W, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998;95(7):1005‐15. - PubMed
Ranganathan 2004
-
- Ranganathan SC, Stocks J, Dezateux C, Bush A, Wade A, Carr S, et al. The evolution of airway function in early childhood following clinical diagnosis of cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2004;169(8):928‐33. - PubMed
Riordan 1989
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245(4922):1066‐73. - PubMed
Rowntree 2003
-
- Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Annals of Human Genetics 2003;67(Pt 5):471‐85. - PubMed
Southern 1996
-
- Southern KW. Gene therapy for cystic fibrosis: current issues. British Journal of Hospital Medicine 1996;55(8):495‐9. - PubMed
Southern 2003
-
- Southern KW, Smyth RL. Design of clinical trials in cystic fibrosis. Lancet 2003;360:978‐84. - PubMed
References to other published versions of this review
Lee 2007
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

