Direct current stimulation induces mGluR5-dependent neocortical plasticity
- PMID: 27315032
- PMCID: PMC8270112
- DOI: 10.1002/ana.24708
Direct current stimulation induces mGluR5-dependent neocortical plasticity
Abstract
Objective: To obtain insights into mechanisms mediating changes in cortical excitability induced by cathodal transcranial direct current stimulation (tDCS).
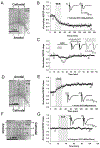
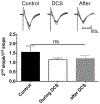
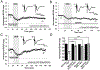
Methods: Neocortical slices were exposed to direct current stimulation (DCS) delivered through Ag/AgCl electrodes over a range of current orientations, magnitudes, and durations. DCS-induced cortical plasticity and its receptor dependency were measured as the change in layer II/III field excitatory postsynaptic potentials by a multielectrode array, both with and without neurotransmitter receptor blockers or allosteric modulators. In vivo, tDCS was delivered to intact mice scalp via surface electrodes. Molecular consequences of DCS in vitro or tDCS in vivo were tested by immunoblot of protein extracted from stimulated slices or the neocortex harvested from stimulated intact mice.
Results: Cathodal DCS in vitro induces a long-term depression (DCS-LTD) of excitatory synaptic strength in both human and mouse neocortical slices. DCS-LTD is abolished with an mGluR5 negative allosteric modulator, mechanistic target of rapamycin (mTOR) inhibitor, and inhibitor of protein synthesis. However, DCS-LTD persists despite either γ-aminobutyric acid type A receptor or N-methyl-D-aspartate receptor inhibition. An mGluR5-positive allosteric modulator, in contrast, transformed transient synaptic depression resultant from brief DCS application into durable DCS-LTD.
Interpretation: We identify a novel molecular pathway by which tDCS modulates cortical excitability, and indicate a capacity for synergistic interaction between tDCS and pharmacologic mGluR5 facilitation. The findings support exploration of cathodal tDCS as a treatment of neurologic conditions characterized by aberrant regional cortical excitability referable to mGluR5-mTOR signaling. Ann Neurol 2016;80:233-246.
© 2016 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
Nothing to report.
Figures
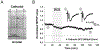
Similar articles
-
Direct Current Stimulation in Cell Culture Systems and Brain Slices-New Approaches for Mechanistic Evaluation of Neuronal Plasticity and Neuromodulation: State of the Art.Cells. 2021 Dec 19;10(12):3583. doi: 10.3390/cells10123583. Cells. 2021. PMID: 34944091 Free PMC article. Review.
-
Drug-Responsive Inhomogeneous Cortical Modulation by Direct Current Stimulation.Ann Neurol. 2020 Sep;88(3):489-502. doi: 10.1002/ana.25822. Epub 2020 Jul 25. Ann Neurol. 2020. PMID: 32542794 Free PMC article.
-
Cocaine Withdrawal Impairs mGluR5-Dependent Long-Term Depression in Nucleus Accumbens Shell Neurons of Both Direct and Indirect Pathways.Mol Neurobiol. 2015 Dec;52(3):1223-1233. doi: 10.1007/s12035-014-8926-z. Epub 2014 Oct 16. Mol Neurobiol. 2015. PMID: 25319571
-
Treatment with direct-current stimulation against cingulate seizure-like activity induced by 4-aminopyridine and bicuculline in an in vitro mouse model.Exp Neurol. 2015 Mar;265:180-92. doi: 10.1016/j.expneurol.2015.02.002. Epub 2015 Feb 12. Exp Neurol. 2015. PMID: 25682917
-
Animal models of transcranial direct current stimulation: Methods and mechanisms.Clin Neurophysiol. 2016 Nov;127(11):3425-3454. doi: 10.1016/j.clinph.2016.08.016. Epub 2016 Sep 10. Clin Neurophysiol. 2016. PMID: 27693941 Free PMC article. Review.
Cited by
-
Transcranial direct current stimulation for post-stroke dysphagia: a meta-analysis.J Neuroeng Rehabil. 2023 Dec 11;20(1):165. doi: 10.1186/s12984-023-01290-w. J Neuroeng Rehabil. 2023. PMID: 38082316 Free PMC article. Review.
-
From adults to pediatrics: A review noninvasive brain stimulation (NIBS) to facilitate recovery from brain injury.Prog Brain Res. 2021;264:287-322. doi: 10.1016/bs.pbr.2021.01.019. Epub 2021 Feb 23. Prog Brain Res. 2021. PMID: 34167660 Free PMC article. Review.
-
Transcranial direct current stimulation leads to faster acquisition of motor skills, but effects are not maintained at retention.PLoS One. 2022 Sep 13;17(9):e0269851. doi: 10.1371/journal.pone.0269851. eCollection 2022. PLoS One. 2022. PMID: 36099260 Free PMC article. Clinical Trial.
-
Robust enhancement of motor sequence learning with 4 mA transcranial electric stimulation.Brain Stimul. 2023 Jan-Feb;16(1):56-67. doi: 10.1016/j.brs.2022.12.011. Epub 2022 Dec 24. Brain Stimul. 2023. PMID: 36574814 Free PMC article. Clinical Trial.
-
Direct Current Stimulation in Cell Culture Systems and Brain Slices-New Approaches for Mechanistic Evaluation of Neuronal Plasticity and Neuromodulation: State of the Art.Cells. 2021 Dec 19;10(12):3583. doi: 10.3390/cells10123583. Cells. 2021. PMID: 34944091 Free PMC article. Review.
References
-
- Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev 2013;11: CD009645. - PubMed
-
- Nitsche MA, Boggio PS, Fregni F, et al. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol 2009;219:14–19. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

