Generalized Liver- and Blood-Derived CD8+ T-Cell Impairment in Response to Cytokines in Chronic Hepatitis C Virus Infection
- PMID: 27315061
- PMCID: PMC4912163
- DOI: 10.1371/journal.pone.0157055
Generalized Liver- and Blood-Derived CD8+ T-Cell Impairment in Response to Cytokines in Chronic Hepatitis C Virus Infection
Abstract
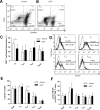
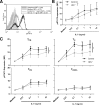
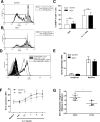
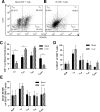
Generalized CD8+ T-cell impairment in chronic hepatitis C virus (HCV) infection and the contribution of liver-infiltrating CD8+ T-cells to the immunopathogenesis of this infection remain poorly understood. It is hypothesized that this impairment is partially due to reduced CD8+ T-cell activity in response to cytokines such as IL-7, particularly within the liver. To investigate this, the phenotype and cytokine responsiveness of blood- and liver-derived CD8+ T-cells from healthy controls and individuals with HCV infection were compared. In blood, IL-7 receptor α (CD127) expression on bulk CD8+ T-cells in HCV infection was no different than controls yet was lower on central memory T-cells, and there were fewer naïve cells. IL-7-induced signalling through phosphorylated STAT5 was lower in HCV infection than in controls, and differed between CD8+ T-cell subsets. Production of Bcl-2 following IL-7 stimulation was also lower in HCV infection and inversely related to the degree of liver fibrosis. In liver-derived CD8+ T-cells, STAT5 activation could not be increased with cytokine stimulation and basal Bcl-2 levels of liver-derived CD8+ T-cells were lower than blood-derived counterparts in HCV infection. Therefore, generalized CD8+ T-cell impairment in HCV infection is characterized, in part, by impaired IL-7-mediated signalling and survival, independent of CD127 expression. This impairment is more pronounced in the liver and may be associated with an increased potential for apoptosis. This generalized CD8+ T-cell impairment represents an important immune dysfunction in chronic HCV infection that may alter patient health.
Conflict of interest statement
Figures
Similar articles
-
Hepatitis C virus core protein reduces CD8+ T-cell proliferation, perforin production and degranulation but increases STAT5 activation.Immunology. 2018 May;154(1):156-165. doi: 10.1111/imm.12882. Epub 2018 Jan 25. Immunology. 2018. PMID: 29266204 Free PMC article.
-
According to Hepatitis C Virus (HCV) Infection Stage, Interleukin-7 Plus 4-1BB Triggering Alone or Combined with PD-1 Blockade Increases TRAF1low HCV-Specific CD8+ Cell Reactivity.J Virol. 2018 Jan 2;92(2):e01443-17. doi: 10.1128/JVI.01443-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093082 Free PMC article.
-
Interleukin-7 augments CD8+ T cells function and promotes viral clearance in chronic hepatitis C virus infection.Cytokine. 2018 Feb;102:26-33. doi: 10.1016/j.cyto.2017.12.014. Epub 2017 Dec 21. Cytokine. 2018. PMID: 29275010
-
Gamma-Chain Receptor Cytokines & PD-1 Manipulation to Restore HCV-Specific CD8+ T Cell Response during Chronic Hepatitis C.Cells. 2021 Mar 3;10(3):538. doi: 10.3390/cells10030538. Cells. 2021. PMID: 33802622 Free PMC article. Review.
-
Chronic hepatitis C in the advanced adult and elderly subjects.Minerva Gastroenterol Dietol. 2009 Jun;55(2):145-57. Minerva Gastroenterol Dietol. 2009. PMID: 19305374 Review.
Cited by
-
Current progress in host innate and adaptive immunity against hepatitis C virus infection.Hepatol Int. 2017 Jul;11(4):374-383. doi: 10.1007/s12072-017-9805-2. Epub 2017 Jun 22. Hepatol Int. 2017. PMID: 28643186 Review.
-
Chronic Hepatitis C Pathogenesis: Immune Response in the Liver Microenvironment and Peripheral Compartment.Front Cell Infect Microbiol. 2021 Aug 3;11:712105. doi: 10.3389/fcimb.2021.712105. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34414132 Free PMC article.
-
Hurdles to the Development of Effective HBV Immunotherapies and HCV Vaccines.Pathog Immun. 2017;2(1):102-125. doi: 10.20411/pai.v2i1.201. Epub 2017 Apr 9. Pathog Immun. 2017. PMID: 28664194 Free PMC article.
-
Chronic Hepatitis C Virus Infection Impairs M1 Macrophage Differentiation and Contributes to CD8+ T-Cell Dysfunction.Cells. 2019 Apr 25;8(4):374. doi: 10.3390/cells8040374. Cells. 2019. PMID: 31027182 Free PMC article.
-
A systematic review of the immuno-inflammatory dysfunction secondary to viral hemorrhagic fevers; Ebola and Lassa fever.PLoS Negl Trop Dis. 2025 Jun 25;19(6):e0013230. doi: 10.1371/journal.pntd.0013230. eCollection 2025 Jun. PLoS Negl Trop Dis. 2025. PMID: 40561148 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

