The Extracellular Surface of the GLP-1 Receptor Is a Molecular Trigger for Biased Agonism
- PMID: 27315480
- PMCID: PMC4912689
- DOI: 10.1016/j.cell.2016.05.023
The Extracellular Surface of the GLP-1 Receptor Is a Molecular Trigger for Biased Agonism
Abstract
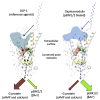
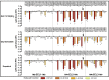
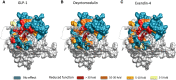
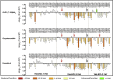
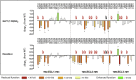
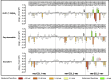
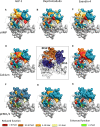
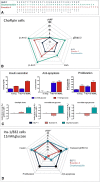
Ligand-directed signal bias offers opportunities for sculpting molecular events, with the promise of better, safer therapeutics. Critical to the exploitation of signal bias is an understanding of the molecular events coupling ligand binding to intracellular signaling. Activation of class B G protein-coupled receptors is driven by interaction of the peptide N terminus with the receptor core. To understand how this drives signaling, we have used advanced analytical methods that enable separation of effects on pathway-specific signaling from those that modify agonist affinity and mapped the functional consequence of receptor modification onto three-dimensional models of a receptor-ligand complex. This yields molecular insights into the initiation of receptor activation and the mechanistic basis for biased agonism. Our data reveal that peptide agonists can engage different elements of the receptor extracellular face to achieve effector coupling and biased signaling providing a foundation for rational design of biased agonists.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Adelhorst K., Hedegaard B.B., Knudsen L.B., Kirk O. Structure-activity studies of glucagon-like peptide-1. J. Biol. Chem. 1994;269:6275–6278. - PubMed
-
- Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. - PubMed
-
- Bergwitz C., Jusseaume S.A., Luck M.D., Jüppner H., Gardella T.J. Residues in the membrane-spanning and extracellular loop regions of the parathyroid hormone (PTH)-2 receptor determine signaling selectivity for PTH and PTH-related peptide. J. Biol. Chem. 1997;272:28861–28868. - PubMed
-
- Black J.W., Leff P. Operational models of pharmacological agonism. Proc. R. Soc. Lond. B Biol. Sci. 1983;220:141–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

