Near-Perfect Synaptic Integration by Nav1.7 in Hypothalamic Neurons Regulates Body Weight
- PMID: 27315482
- PMCID: PMC4912688
- DOI: 10.1016/j.cell.2016.05.019
Near-Perfect Synaptic Integration by Nav1.7 in Hypothalamic Neurons Regulates Body Weight
Abstract
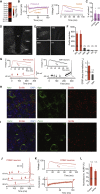
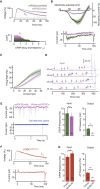
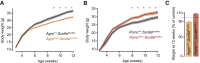
Neurons are well suited for computations on millisecond timescales, but some neuronal circuits set behavioral states over long time periods, such as those involved in energy homeostasis. We found that multiple types of hypothalamic neurons, including those that oppositely regulate body weight, are specialized as near-perfect synaptic integrators that summate inputs over extended timescales. Excitatory postsynaptic potentials (EPSPs) are greatly prolonged, outlasting the neuronal membrane time-constant up to 10-fold. This is due to the voltage-gated sodium channel Nav1.7 (Scn9a), previously associated with pain-sensation but not synaptic integration. Scn9a deletion in AGRP, POMC, or paraventricular hypothalamic neurons reduced EPSP duration, synaptic integration, and altered body weight in mice. In vivo whole-cell recordings in the hypothalamus confirmed near-perfect synaptic integration. These experiments show that integration of synaptic inputs over time by Nav1.7 is critical for body weight regulation and reveal a mechanism for synaptic control of circuits regulating long term homeostatic functions.
Copyright © 2016 MRC Laboratory of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Slowly Building Excitement.Cell. 2016 Jun 16;165(7):1568-1569. doi: 10.1016/j.cell.2016.06.005. Cell. 2016. PMID: 27315473
References
-
- Balthasar N., Coppari R., McMinn J., Liu S.M., Lee C.E., Tang V., Kenny C.D., McGovern R.A., Chua S.C., Jr., Elmquist J.K., Lowell B.B. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

