Cold/menthol TRPM8 receptors initiate the cold-shock response and protect germ cells from cold-shock-induced oxidation
- PMID: 27317670
- PMCID: PMC5001517
- DOI: 10.1096/fj.201600257R
Cold/menthol TRPM8 receptors initiate the cold-shock response and protect germ cells from cold-shock-induced oxidation
Abstract
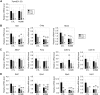
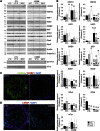
Testes of most male mammals present the particularity of being externalized from the body and are consequently slightly cooler than core body temperature (4-8°C below). Although, hypothermia of the testis is known to increase germ cells apoptosis, little is known about the underlying molecular mechanisms, including cold sensors, transduction pathways, and apoptosis triggers. In this study, using a functional knockout mouse model of the cold and menthol receptors, dubbed transient receptor potential melastatine 8 (TRPM8) channels, we found that TRPM8 initiated the cold-shock response by differentially modulating cold- and heat-shock proteins. Besides, apoptosis of germ cells increased in proportion to the cooling level in control mice but was independent of temperature in knockout mice. We also observed that the rate of germ cell death correlated positively with the reactive oxygen species level and negatively with the expression of the detoxifying enzymes. This result suggests that the TRPM8 sensor is a key determinant of germ cell fate under hypothermic stimulation.-Borowiec, A.-S., Sion, B., Chalmel, F., Rolland, A. D., Lemonnier, L., De Clerck, T., Bokhobza, A., Derouiche, S., Dewailly, E., Slomianny, C., Mauduit, C., Benahmed, M., Roudbaraki, M., Jégou, B., Prevarskaya, N., Bidaux, G. Cold/menthol TRPM8 receptors initiate the cold-shock response and protect germ cells from cold-shock-induced oxidation.
Keywords: apoptosis; hypothermia; spermatogenesis.
© The Author(s).
Figures




References
-
- Chowdhury A. K., Steinberger E. (1970) Early changes in the germinal epithelium of rat testes following exposure to heat. J. Reprod. Fertil. 22, 205–212 - PubMed
-
- Macdonald J., Harrison R. G. (1954) Effect of low temperatures on rat spermatogenesis. Fertil. Steril. 5, 205–216 - PubMed
-
- Blanco-Rodríguez J., Martínez-García C. (1997) Mild hypothermia induces apoptosis in rat testis at specific stages of the seminiferous epithelium. J. Androl. 18, 535–539 - PubMed
-
- Zhang Z., Short R. V., Meehan T., De Kretser D. M., Renfree M. B., Loveland K. L. (2004) Functional analysis of the cooled rat testis. J. Androl. 25, 57–68 - PubMed
-
- Yazawa T., Nakayama Y., Fujimoto K., Matsuda Y., Abe K., Kitano T., Abé S., Yamamoto T. (2003) Abnormal spermatogenesis at low temperatures in the Japanese red-bellied newt, Cynops pyrrhogaster: possible biological significance of the cessation of spermatocytogenesis. Mol. Reprod. Dev. 66, 60–66 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

