Randomized evaluation of fibrinogen vs placebo in complex cardiovascular surgery (REPLACE): a double-blind phase III study of haemostatic therapy
- PMID: 27317703
- PMCID: PMC4913420
- DOI: 10.1093/bja/aew169
Randomized evaluation of fibrinogen vs placebo in complex cardiovascular surgery (REPLACE): a double-blind phase III study of haemostatic therapy
Abstract
Background: Single-dose human fibrinogen concentrate (FCH) might have haemostatic benefits in complex cardiovascular surgery.
Methods: Patients undergoing elective aortic surgery requiring cardiopulmonary bypass were randomly assigned to receive FCH or placebo. Study medication was administered to patients with a 5 min bleeding mass of 60-250 g after separation from bypass and surgical haemostasis. A standardized algorithm for allogeneic blood product transfusion was followed if bleeding continued after study medication.
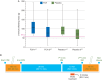
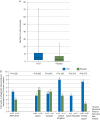
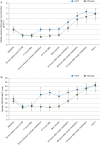
Results: 519 patients from 34 centres were randomized, of whom 152 (29%) met inclusion criteria for study medication. Median (IQR) pretreatment 5 min bleeding mass was 107 (76-138) and 91 (71-112) g in the FCH and placebo groups, respectively (P=0.13). More allogeneic blood product units were administered during the first 24 h after FCH, 5.0 (2.0-11.0), when compared with placebo, 3.0 (0.0-7.0), P=0.026. Fewer patients avoided transfusion in the FCH group (15.4%) compared with placebo (28.4%), P=0.047. The FCH immediately increased plasma fibrinogen concentration and fibrin-based clot strength. Adverse event rates were comparable in each group.
Conclusions: Human fibrinogen concentrate was associated with increased allogeneic blood product transfusion, an unexpected finding contrary to previous studies. Human fibrinogen concentrate may not be effective in this setting when administered according to 5-minute bleeding mass. Low bleeding rates and normal-range plasma fibrinogen concentrations before study medication, and variability in adherence to the complex transfusion algorithm, may have contributed to these results.
Clinical trial registration: ClinicalTrials.gov identifier no. NCT01475669; EudraCT trial no. 2011-002685-20.
Keywords: blood, coagulation; fibrinogen; haemorrhage; surgery, cardiovascular.
© The Author 2016. Published by Oxford University Press on behalf of the British Journal of Anaesthesia. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Supplementary fibrinogen in the management of bleeding: re-evaluation of data from clinical trials.Br J Anaesth. 2018 Feb;120(2):407-409. doi: 10.1016/j.bja.2017.11.092. Epub 2017 Dec 5. Br J Anaesth. 2018. PMID: 29406192 No abstract available.
References
-
- Sniecinski RM, Levy JH. Bleeding and management of coagulopathy. J Thorac Cardiovasc Surg 2011; 142: 662–7 - PubMed
-
- Levy JH, Szlam F, Tanaka KA, Sniecienski RM. Fibrinogen and hemostasis: a primary hemostatic target for the management of acquired bleeding. Anesth Analg 2012; 114: 261–74 - PubMed
-
- Tanaka KA, Esper S, Bolliger D. Perioperative factor concentrate therapy. Br J Anaesth 2013; 111(Suppl 1): i35–49 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

