Skeletal muscle and plasma concentrations of cefazolin during complex paediatric spinal surgery
- PMID: 27317707
- PMCID: PMC4913389
- DOI: 10.1093/bja/aew032
Skeletal muscle and plasma concentrations of cefazolin during complex paediatric spinal surgery
Abstract
Background: Surgical site infections (SSIs) can have devastating consequences for children who undergo spinal instrumentation. Prospective evaluations of prophylactic cefazolin in this population are limited. The purpose of this study was to describe the pharmacokinetics and skeletal muscle disposition of prophylactic cefazolin in a paediatric population undergoing complex spinal surgery.
Methods: This prospective pharmacokinetic study included 17 children with adolescent idiopathic scoliosis undergoing posterior spinal fusion, with a median age of 13.8 [interquartile range (IQR) 13.4-15.4] yr and a median weight of 60.6 (IQR 50.8-66.0) kg. A dosing strategy consistent with published guidelines was used. Serial plasma and skeletal muscle microdialysis samples were obtained during the operative procedure and unbound cefazolin concentrations measured. Non-compartmental pharmacokinetic analyses were performed. The amount of time that the concentration of unbound cefazolin exceeded the minimal inhibitory concentration for bacterial growth for selected SSI pathogens was calculated.
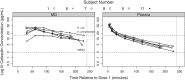
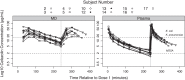
Results: Skeletal muscle concentrations peaked at a median of 37.6 (IQR 26.8-40.0) µg ml(-1) within 30-60 min after the first cefazolin 30 mg kg(-1) dose. For patients who received a second 30 mg kg(-1) dose, the peak concentrations reached a median of 40.5 (IQR 30.8-45.7) µg ml(-1) within 30-60 min. The target cefazolin concentrations for SSI prophylaxis for meticillin-sensitive Staphylococcus aureus (MSSA) and Gram-negative pathogens were exceeded in skeletal muscle 98.9 and 58.3% of the intraoperative time, respectively.
Conclusions: For children with adolescent idiopathic scoliosis undergoing posterior spinal fusion, the cefazolin dosing strategy used in this study resulted in skeletal muscle concentrations that were likely not to be effective for intraoperative SSI prophylaxis against Gram-negative pathogens.
Keywords: cefazolin; microdialysis; paediatric; scoliosis; surgery, spinal.
© The Author 2016. Published by Oxford University Press on behalf of the British Journal of Anaesthesia. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Skeletal muscle and plasma concentrations of cefazolin.Br J Anaesth. 2016 Jul;117(1):3-5. doi: 10.1093/bja/aew147. Br J Anaesth. 2016. PMID: 27317702 Free PMC article. No abstract available.
References
-
- Labbé AC, Demers AM, Rodrigues R, Arlet V, Tanguay K, Moore L. Surgical-site infection following spinal fusion: a case control study in a children's hospital. Infect Control Hosp Epidemiol 2003; 24: 591–5 - PubMed
-
- Hedequist D, Haugen A, Hresko T, Emans J. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine (Phila Pa 1976) 2009; 34: 60–4 - PubMed
-
- Ho C, Skaggs DL, Weiss JM, Tolo VT. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine (Phila Pa 1976) 2007; 32: 2739–44 - PubMed
-
- Linam WM, Margolis PA, Staat MA et al. . Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol 2009; 30: 109–16 - PubMed
-
- Ali MHM, Koutharawu DN, Miller F et al. . Operative and clinical markers of deep wound infection after spine fusion in children with cerebral palsy. J Pediatr Orthop 2010; 30: 851–7 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

